Label: FOSCARNET SODIUM injection, solution
- NDC Code(s): 23155-771-31, 23155-771-41
- Packager: Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
RENAL IMPAIRMENT IS THE MAJOR TOXICITY OF FOSCARNET SODIUM INJECTION. FREQUENT MONITORING OF SERUM CREATININE, WITH DOSE ADJUSTMENT FOR CHANGES IN RENAL FUNCTION, AND ADEQUATE HYDRATION WITH ADMINISTRATION OF FOSCARNET SODIUM INJECTION IS IMPERATIVE. (See ADMINISTRATION section; Hydration.)
SEIZURES, RELATED TO ALTERATIONS IN PLASMA MINERALS AND ELECTROLYTES, HAVE BEEN ASSOCIATED WITH FOSCARNET SODIUM INJECTION TREATMENT. THEREFORE, PATIENTS MUST BE CAREFULLY MONITORED FOR SUCH CHANGES AND THEIR POTENTIAL SEQUELAE. MINERAL AND ELECTROLYTE SUPPLEMENTATION MAY BE REQUIRED.
FOSCARNET SODIUM INJECTION IS INDICATED FOR USE ONLY IN IMMUNOCOMPROMISED PATIENTS WITH CMV RETINITIS AND MUCOCUTANEOUS ACYCLOVIR-RESISTANT HSV INFECTIONS. (See INDICATIONS section).
Close -
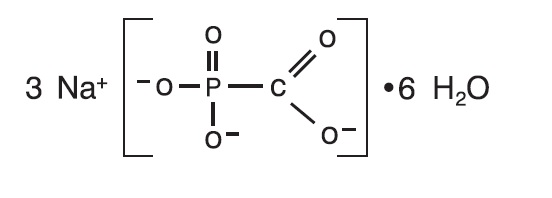
DESCRIPTIONThe chemical name of foscarnet sodium is phosphonoformic acid, trisodium salt, hexahydrate. Foscarnet sodium is a white to almost white, crystalline powder containing 6 equivalents of water of ...
-
VIROLOGYMechanism of Action - Foscarnet exerts its antiviral activity by a selective inhibition at the pyrophosphate binding site on virus-specific DNA polymerases at concentrations that do not affect ...
-
CLINICAL PHARMACOLOGYPharmacokinetics - The pharmacokinetics of foscarnet has been determined after administration as an intermittent intravenous infusion during induction therapy in AIDS patients with CMV ...
-
INDICATIONSCMV Retinitis - Foscarnet sodium injection is indicated for the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS). Combination therapy with foscarnet sodium ...
-
CONTRAINDICATIONSFoscarnet sodium injection is contraindicated in patients with clinically significant hypersensitivity to foscarnet sodium.
-
WARNINGSRenal Impairment - THE MAJOR TOXICITY OF FOSCARNET SODIUM INJECTION IS RENAL IMPAIRMENT (see ADVERSE REACTIONS section). Renal impairment is most likely to become clinically evident during the ...
-
PRECAUTIONSGeneral - Care must be taken to infuse solutions containing foscarnet sodium only into veins with adequate blood flow to permit rapid dilution and distribution to avoid local irritation (see ...
-
ADVERSE REACTIONSTHE MAJOR TOXICITY OF FOSCARNET SODIUM INJECTION IS RENAL IMPAIRMENT (see WARNINGS section). Approximately 33% of 189 patients with AIDS and CMV retinitis who received foscarnet sodium injection ...
-
OVERDOSAGEIn controlled clinical trials performed in the United States, overdosage with foscarnet sodium injection was reported in 10 out of 189 patients. All 10 patients experienced adverse events and all ...
-
DOSAGE AND ADMINISTRATIONCAUTION - DO NOT ADMINISTER FOSCARNET SODIUM INJECTION BY RAPID OR BOLUS INTRAVENOUS INJECTION. THE TOXICITY OF FOSCARNET SODIUM INJECTION MAY BE INCREASED AS A RESULT OF EXCESSIVE PLASMA LEVELS ...
-
PATIENT MONITORINGThe majority of patients will experience some decrease in renal function due to foscarnet sodium injection administration. Therefore it is recommended that creatinine clearance, either measured ...
-
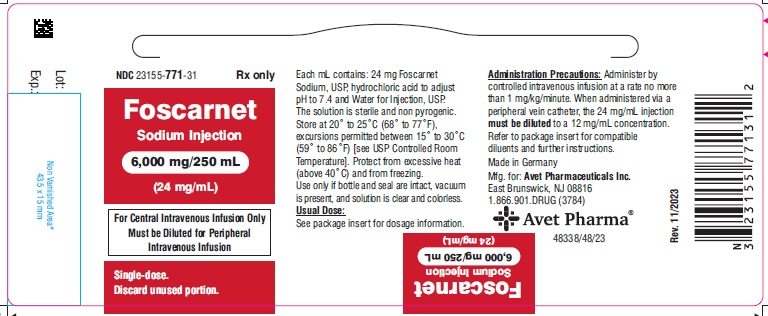
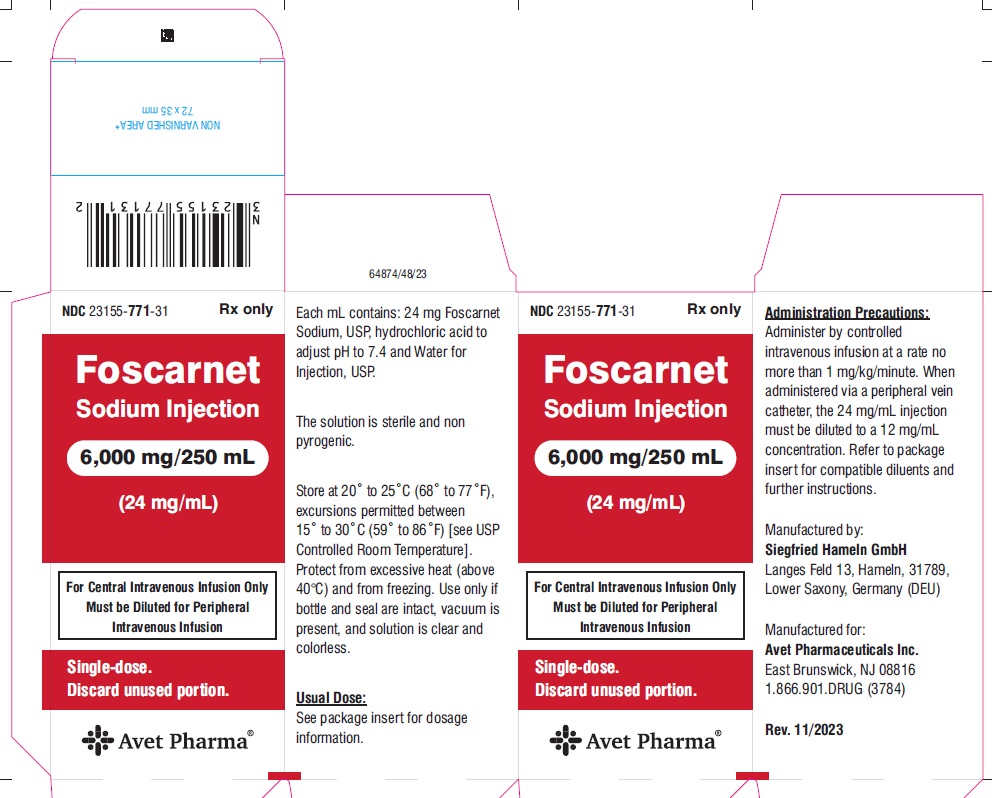
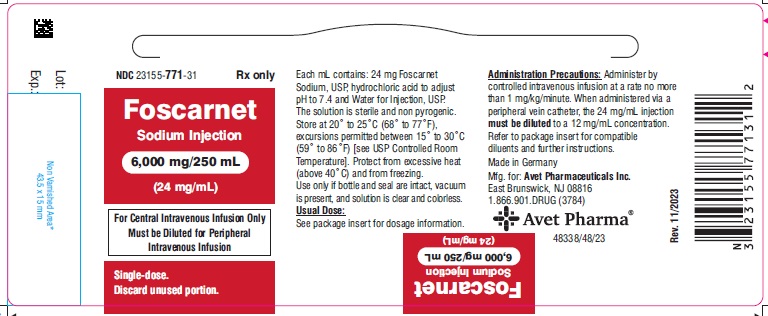
HOW SUPPLIEDFoscarnet Sodium Injection, 24 mg/mL for intravenous infusion, is supplied in 250 mL glass bottles containing 6,000 mg foscarnet sodium (24 mg/mL) as follows: NDC 23155-771-31 250 mL bottle, 1 ...
-
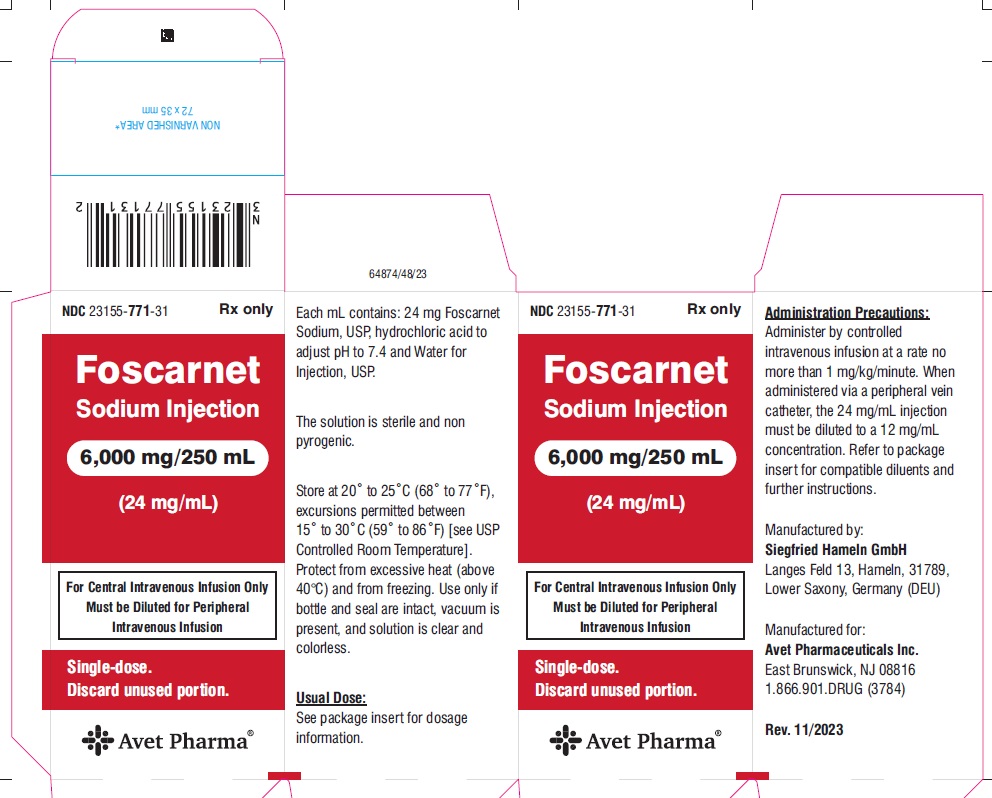
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 23155-771-31 - Foscarnet Sodium Injection - 6,000 mg/250 mL - (24 mg/mL) For Central Intravenous Infusion Only - Must be Diluted for Peripheral - Intravenous Infusion - Single-dose. Discard ...
-
INGREDIENTS AND APPEARANCEProduct Information