Label: EPINEPHRINE CHLORIDE SOLUTION- epinephrine nasal solution solution
- NDC Code(s): 54288-123-01
- Packager: BPI Labs, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
-
3 WARNINGS AND PRECAUTIONS
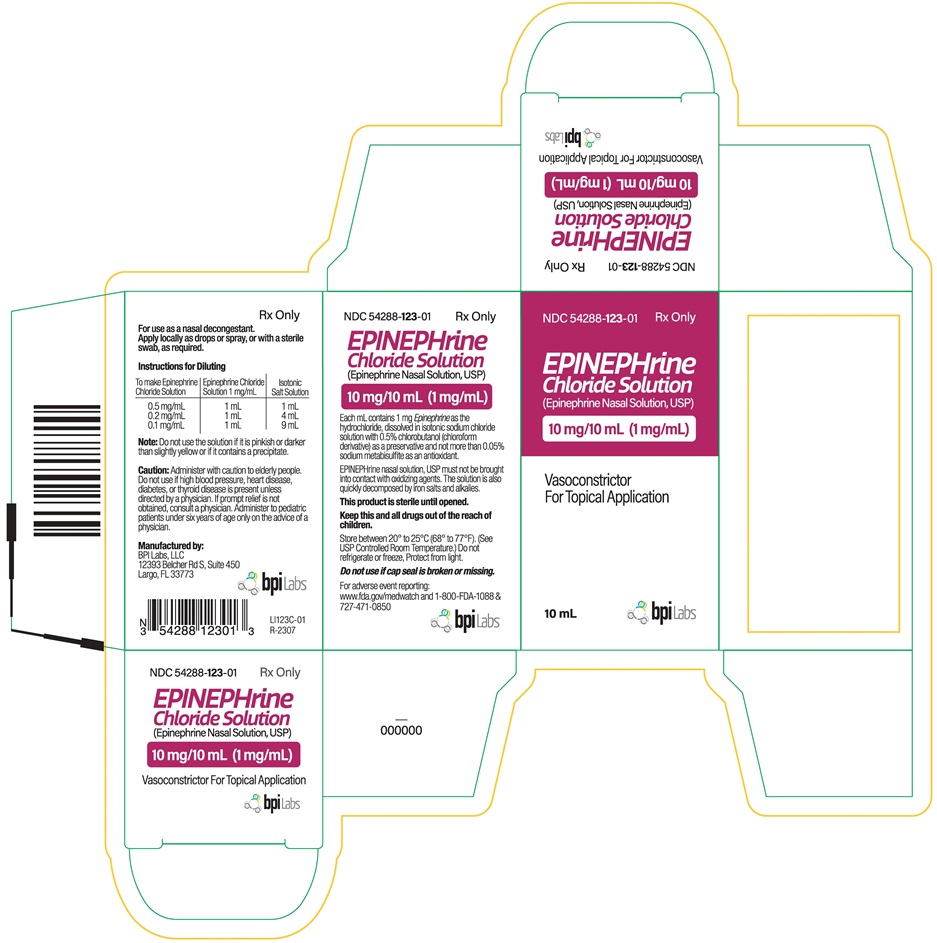
Caution: Administer with caution to elderly people. Do not use if high blood pressure, heart disease, diabetes, or thyroid disease is present unless directed by a physician. If prompt relief is not obtained, consult a physician. Administer to pediatric patients under six years of age only on the advice of a physician.
-
4 DESCRIPTION
Each mL contains 1 mg Epinephrine as the hydrochloride, dissolved in isotonic sodium chloride solution with 0.5% chlorobutanol (chloroform derivative) as a preservative and not more than 0.05% sodium metabisulfite as an antioxidant.
EPINEPHrine nasal solution, USP must not be brought into contact with oxidizing agents.The solution is also quickly decomposed by iron salts and alkalies.
- 5 ADVERSE REACTIONS
-
6 HOW SUPPLIED/STORAGE AND HANDLING
Each carton contains one vial of 10 mg/10 mL (1 mg/mL) of Epinephrine Chloride Solution (Epinephrine Nasal Solution,USP) in an amber glass vial.
NDC 54288-123-01 10 mL Vial
This product is sterile until opened.
Keep this and all drugs out of the reach of children.
Store between 20° to 25°C (68° to 77°F). (See USP Controlled Room Temperature.) Do not refrigerate or freeze, Protect from light.
Do not use if cap seal is broken or missing.
Manufactured by:
BPI Labs, LLC
12393 Belcher Rd S, Suite 450
Largo, FL 33773
- PACKAGE LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EPINEPHRINE CHLORIDE SOLUTION
epinephrine nasal solution solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54288-123 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE HYDROCHLORIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOTONIC SODIUM CHLORIDE SOLUTION (UNII: VR5Y7PDT5W) SODIUM METABISULFITE (UNII: 4VON5FNS3C) CHLOROBUTANOL (UNII: HM4YQM8WRC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54288-123-01 10 mL in 1 BOX; Type 0: Not a Combination Product 09/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/30/2020 Labeler - BPI Labs, LLC (078627620) Establishment Name Address ID/FEI Business Operations BPI Labs, LLC 078627620 manufacture(54288-123) , label(54288-123)