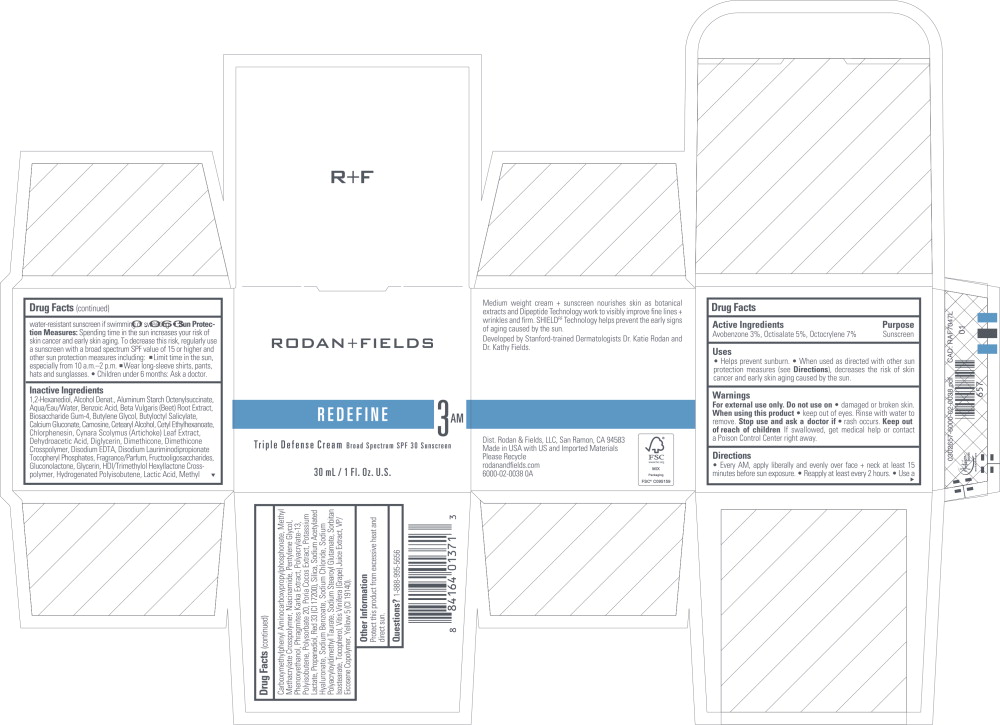
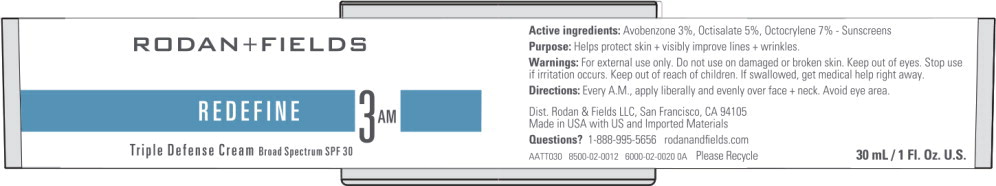
Label: REDEFINE TRIPLE DEFENSE- avobenzone, octisalate, octocrylene cream
- NDC Code(s): 14222-2251-1, 14222-2251-2, 14222-2251-3
- Packager: Rodan & Fields
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
-
Uses
- Helps prevent sunburn.
- When used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Every AM, apply liberally and evenly over face + neck at least 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and ear y skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m -2 p.m.
- Wear long-sleeve shirts, pants, hats and sunglasses.
- Children under 6 months: Ask a doctor.
-
Inactive Ingredients
1,2-Hexanediol, Alcohol Denat., Aluminum Starch Octenylsuccinate, Aqua/Eau/Water, Benzoic Acid, Beta Vulgaris (Beet) Root Extract, Biosaccharide Gum-4, Butylene Glycol, Butyloctyl Salicylate, Calcium Gluconate, Carnosine, Cetearyl Alcohol, Cetyl Ethylhexanoate, Chlorphenesin, Cynara Scolymus (Artichoke) Leaf Extract, Dehydroacetic Acid, Diglycerin, Dimethicone, Dimethicone Crosspolymer, Disodium EDTA, Disodium Lauriminodipropionate Tocopheryl Phosphates, Fragrance/Parfum, Fructooligosaccharides, Gluconolactone, Glycerin, HDI/Trimethylol Hexyllactone Crosspolymer, Hydrogenated Polyisobutene, Lactic Acid, Methyl Carboxymethylphenyl Aminocarboxypropylphosphonate, Methyl Methacrylate Crosspolymer, Niacinamide, Pentylene Glycol, Phenoxyethanol, Phragmites Karka Extract, Polyacrylate-13, Polyisobutene, Polysorbate 20, Poria Cocos Extract, Potassium Lactate, Propanediol, Red 33 (Cl 17200), Silica, Sodium Acetylated Hyaluronate, Sodium Benzoate, Sodium Chloride, Sodium Polyacryloyldimethyl Taurate, Sodium Stearoyl Glutamate, Sorbitan Isostearate, Tocopherol, Vitis Vinifera (Grape) Juice Extract, VP/Eicosene Copolymer, Yellow 5 (Cl 19140).
- Other Information
- Questions?
- Principal Display Panel - 30 mL Carton Label
- Principal Display Panel - 30 mL Jar Label
-
INGREDIENTS AND APPEARANCE
REDEFINE TRIPLE DEFENSE
avobenzone, octisalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2251 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.07 g in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.05 g in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.03 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NIACINAMIDE (UNII: 25X51I8RD4) PENTYLENE GLYCOL (UNII: 50C1307PZG) EICOSYL POVIDONE (UNII: XQQ9MKE2BJ) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) DIGLYCERIN (UNII: 3YC120743U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) BETA VULGARIS (UNII: 4G174V5051) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) CHLORPHENESIN (UNII: I670DAL4SZ) PROPANEDIOL (UNII: 5965N8W85T) CARNOSINE (UNII: 8HO6PVN24W) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) POLYISOBUTYLENE (2300 MW) (UNII: DSQ2V1DD1K) POTASSIUM LACTATE (UNII: 87V1KMK4QV) DISODIUM LAURIMINODIPROPIONATE TOCOPHERYL PHOSPHATES (UNII: 0K5Y9U1P6M) CYNARA SCOLYMUS LEAF (UNII: B71UA545DE) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM CHLORIDE (UNII: 451W47IQ8X) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) GLUCONOLACTONE (UNII: WQ29KQ9POT) SODIUM BENZOATE (UNII: OJ245FE5EU) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 33 (UNII: 9DBA0SBB0L) BENZOIC ACID (UNII: 8SKN0B0MIM) CALCIUM GLUCONATE (UNII: SQE6VB453K) DEHYDROACETIC ACID (UNII: 2KAG279R6R) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2251-1 1 in 1 CARTON 10/18/2020 1 30 mL in 1 JAR; Type 0: Not a Combination Product 2 NDC:14222-2251-2 1 in 1 CARTON 06/01/2021 2 7 mL in 1 JAR; Type 0: Not a Combination Product 3 NDC:14222-2251-3 2 mL in 1 PACKET; Type 0: Not a Combination Product 04/12/2022 01/05/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/18/2020 Labeler - Rodan & Fields (051659584)