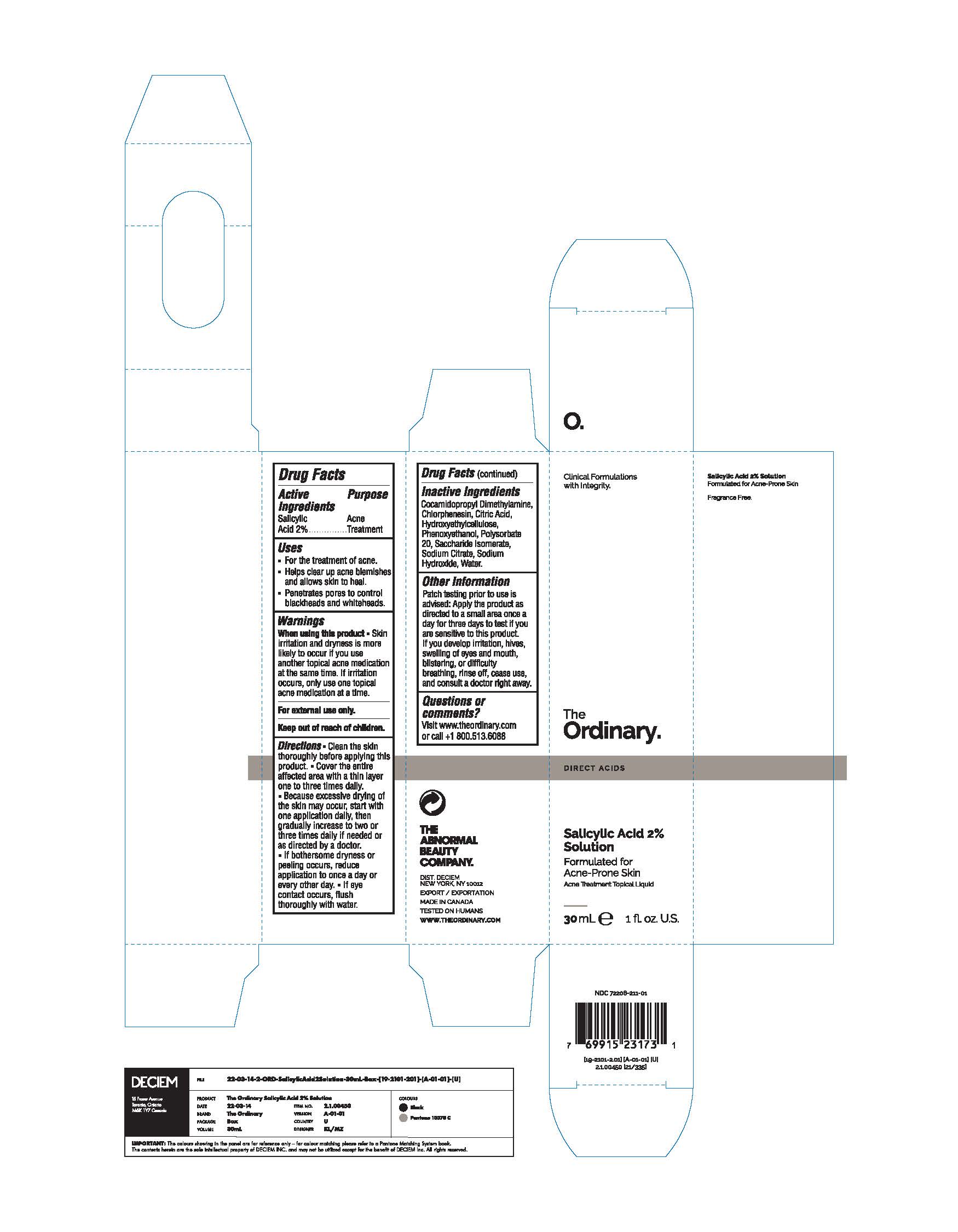
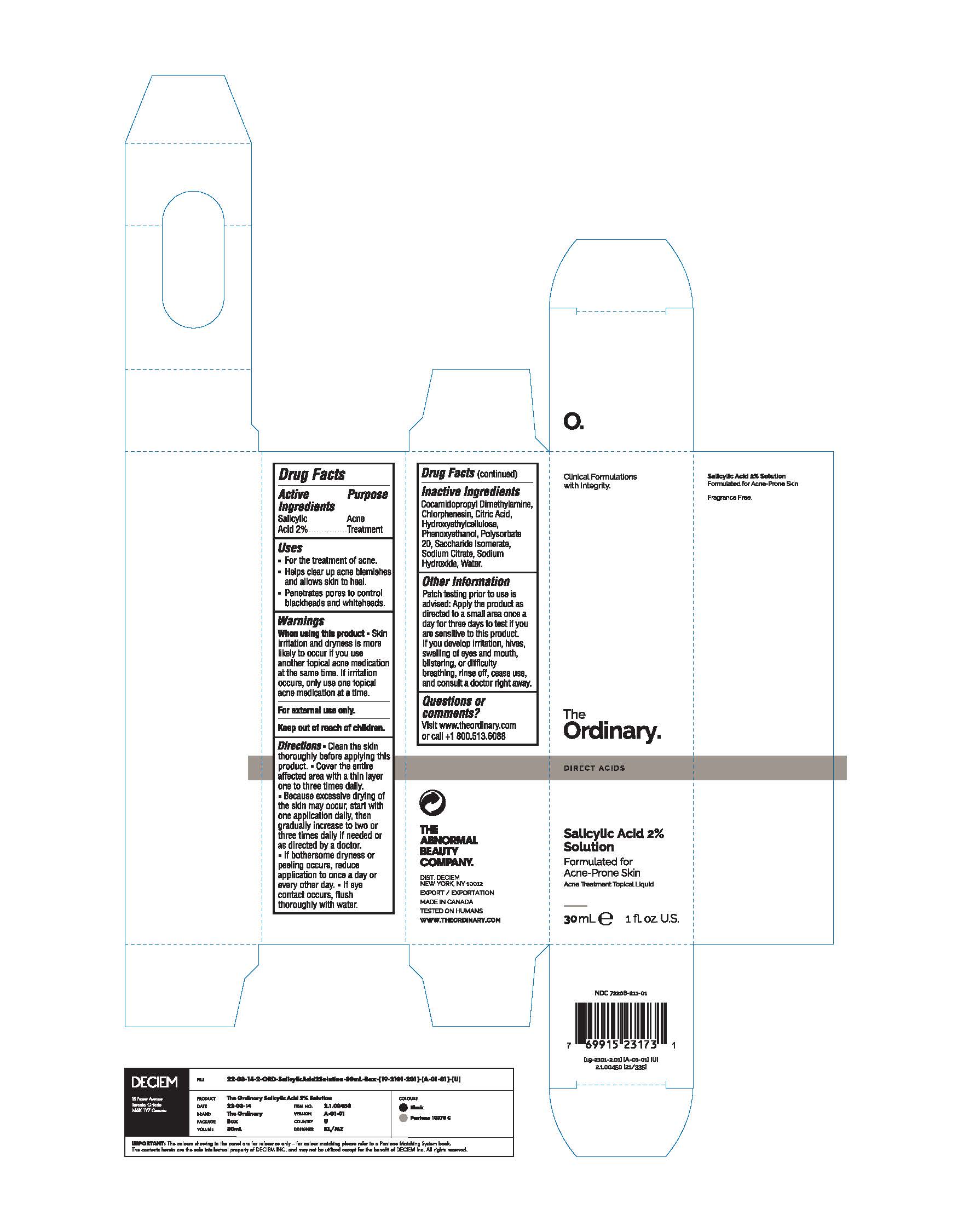
Label: THE ORDINARY SALICYLIC ACID 2% SOLUTION- salicylic acid liquid
- NDC Code(s): 72208-211-01
- Packager: Deciem
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- DOSAGE & ADMINISTRATION
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THE ORDINARY SALICYLIC ACID 2% SOLUTION
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72208-211 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength SACCHARIDE ISOMERATE (UNII: W8K377W98I) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) COCAMIDOPROPYL DIMETHYLAMINE (UNII: L36BM7DG2T) CHLORPHENESIN (UNII: I670DAL4SZ) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72208-211-01 1 in 1 BOX 02/25/2022 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/15/2022 Labeler - Deciem (203133665) Establishment Name Address ID/FEI Business Operations HK Kolmar Canada, Inc 243501959 analysis(72208-211) , manufacture(72208-211) , pack(72208-211) Establishment Name Address ID/FEI Business Operations Crystal Claire Cosmetics Inc. 205493484 manufacture(72208-211) , pack(72208-211) , analysis(72208-211)