Label: MELOXICAM tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 71335-1618-0, 71335-1618-1, 71335-1618-2, 71335-1618-3, view more - Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 63629-7966
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 10, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MELOXICAM TABLETS USP. safely and effectively. See full prescribing information for MELOXICAM TABLETS USP. MELOXICAM Tablets USP ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS - Cardiovascular Thrombotic Events - Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious ...Close
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
Cardiovascular Thrombotic Events
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use [see Warnings and Precautions (5.1) ].
- Meloxicam tablets are contraindicated in the setting of coronary artery bypass graft (CABG) surgery [see Contraindications (4) and Warnings and Precautions (5.1) ].
Gastrointestinal Bleeding, Ulceration, and Perforation
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events [see Warnings and Precautions (5.2) ].
-
1 INDICATIONS AND USAGE1.1 Osteoarthritis (OA) Meloxicam tablets are indicated for relief of the signs and symptoms of osteoarthritis [see Clinical Studies (14.1)]. 1.2 Rheumatoid Arthritis (RA) Meloxicam tablets ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Instructions - Carefully consider the potential benefits and risks of Meloxicam tablets and other treatment options before deciding to use Meloxicam tablets. Use the lowest ...
-
3 DOSAGE FORMS AND STRENGTHSMeloxicam Tablets USP: 7.5 mg: Light yellow, round flat beveled edged, tablet with U & L debossed on one side and 7.5 debossed centrally on the other side - 15 mg: Light yellow, capsule shaped ...
-
4 CONTRAINDICATIONSMeloxicam tablets are contraindicated in the following patients: Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to meloxicam or any components of the drug ...
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiovascular Thrombotic Events - Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling: Cardiovascular Thrombotic Events [see Boxed Warning and Warnings and Precautions (5.1) ] GI ...
-
7 DRUG INTERACTIONSSee Table 3 for clinically significant drug interactions with meloxicam. See also Warnings and Precautions (5.2, 5.6, 5.11) and Clinical Pharmacology (12.3). Table 3 Clinically Significant ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Use of NSAIDs, including Meloxicam, during the third trimester of pregnancy increases the risk of premature closure of the fetal ductus arteriosus. Avoid use of ...
-
10 OVERDOSAGESymptoms following acute NSAID overdosages have been typically limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which have been generally reversible with supportive care ...
-
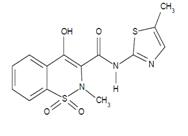
11 DESCRIPTIONMeloxicam Tablets USP are a nonsteroidal anti-inflammatory drug (NSAID). Each tablet contains 7.5 mg or 15 mg meloxicam for oral administration. Meloxicam is chemically designated as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Meloxicam has analgesic, anti-inflammatory, and antipyretic properties. The mechanism of action of Meloxicam, like that of other NSAIDs, is not completely understood ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - There was no increase in tumor incidence in long-term carcinogenicity studies in rats (104 weeks) and mice (99 weeks ...
-
14 CLINICAL STUDIES14.1 Osteoarthritis and Rheumatoid Arthritis - The use of Meloxicam for the treatment of the signs and symptoms of osteoarthritis of the knee and hip was evaluated in a 12-week, double-blind ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNDC: 71335-1618-1: 30 Tablets in a BOTTLE - NDC: 71335-1618-2: 60 Tablets in a BOTTLE - NDC: 71335-1618-3: 90 Tablets in a BOTTLE - NDC: 71335-1618-4: 14 Tablets in a ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide) that accompanies each prescription dispensed. Additional Medication Guides can be obtained by calling Unichem at ...
-
SPL MEDGUIDEMedication Guide for Nonsteroidal Anti-inflammatory Drugs (NSAIDs) What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs ...
-
PRINCIPAL DISPLAY PANELMeloxicam 15mg Tablet
-
INGREDIENTS AND APPEARANCEProduct Information