Label: NEOSTIGMINE METHYLSULFATE injection
- NDC Code(s): 31722-994-31, 31722-995-31
- Packager: Camber Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 22, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NEOSTIGMINE METHYLSULFATE INJECTION safely and effectively. See full prescribing information for NEOSTIGMINE METHYLSULFATE INJECTION ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS & USAGENeostigmine methylsulfate injection is a cholinesterase inhibitor indicated for the reversal of the effects of non-depolarizing neuromuscular blocking agents after surgery.
-
2 DOSAGE & ADMINISTRATION2.1. Important Dosage Information - Neostigmine methylsulfate injection should be administered by trained healthcare providers familiar with the use, actions, characteristics, and complications ...
-
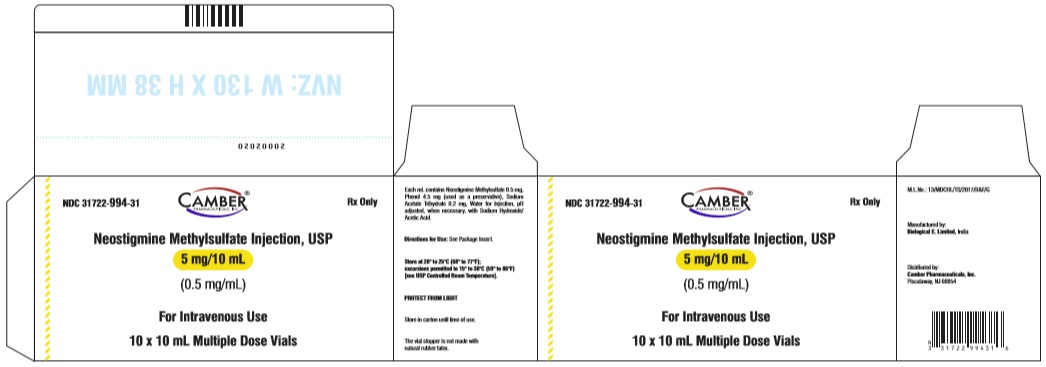
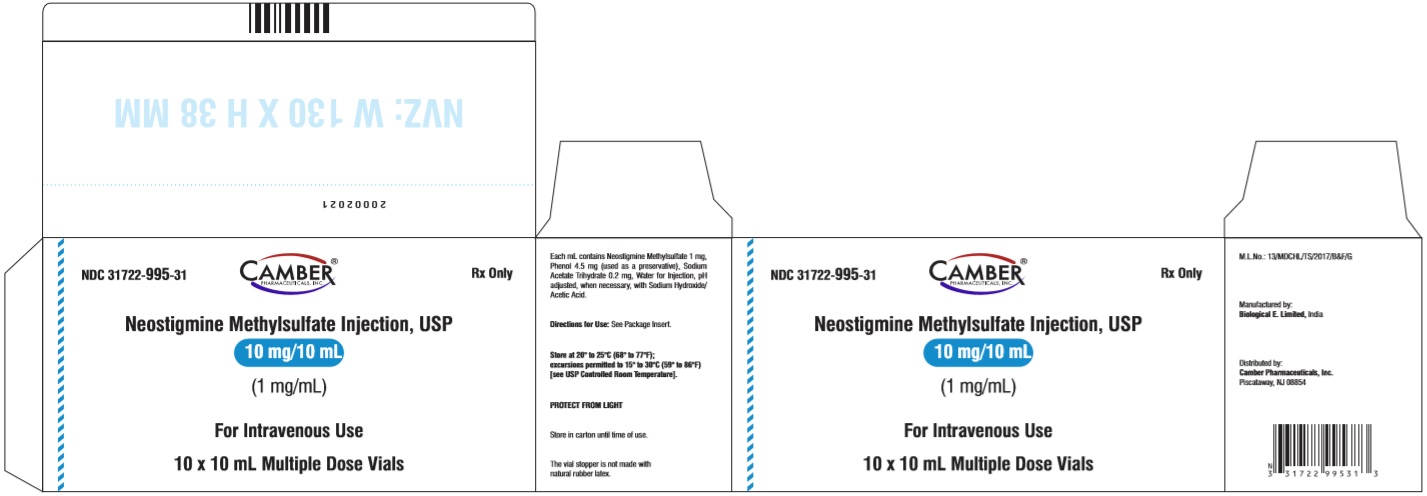
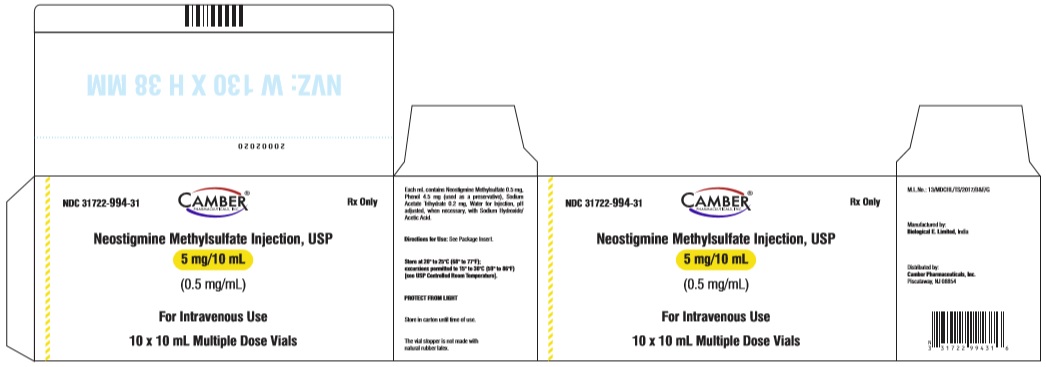
3 DOSAGE FORMS & STRENGTHSNeostigmine methylsulfate injection, USP is available as Injection: 0.5 mg/mL, 5 mg of neostigmine methylsulfate in 10 mL multiple-dose vials - Injection: 1 mg/mL, 10 mg of neostigmine ...
-
4 CONTRAINDICATIONSNeostigmine methylsulfate injection is contraindicated in patients with: known hypersensitivity to neostigmine methylsulfate (known hypersensitivity reactions have included urticaria ...
-
5 WARNINGS AND PRECAUTIONS5.1. Bradycardia - Neostigmine has been associated with bradycardia. Atropine sulfate or glycopyrrolate should be administered prior to neostigmine methylsulfate injection to lessen the risk of ...
-
6 ADVERSE REACTIONS6.1. Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSThe pharmacokinetic interaction between neostigmine methylsulfate and other drugs has not been studied. Neostigmine methylsulfate is metabolized by microsomal enzymes in the liver. Use with ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate or well-controlled studies of neostigmine methylsulfate injection in pregnant women. It is not known whether neostigmine methylsulfate ...
-
10 OVERDOSAGEMuscarinic symptoms (nausea, vomiting, diarrhea, sweating, increased bronchial and salivary secretions, and bradycardia) may appear with overdosage of neostigmine methylsulfate injection ...
-
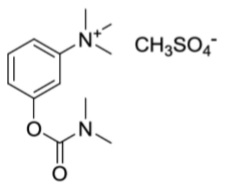
11 DESCRIPTIONNeostigmine methylsulfate, a cholinesterase inhibitor, is (m-hydroxyphenyl) trimethylammonium methylsulfate dimethylcarbamate. The structural formula is: Neostigmine methylsulfate USP is a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Neostigmine methylsulfate is a competitive cholinesterase inhibitor. By reducing the breakdown of acetylcholine, neostigmine methylsulfate induces an increase in ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility - Carcinogenesis: Long-term animal studies have not been performed to evaluate the carcinogenic potential of neostigmine ...
-
14 CLINICAL STUDIESThe evidence for the efficacy of neostigmine methylsulfate for the reversal of the effects of non-depolarizing neuromuscular blocking agents after surgery is derived from the published literature ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNeostigmine methylsulfate injection, USP is a clear, colourless solution free from visible particles in a glass vial. It is available as follows: NDC No. Strength - Vial ...
-
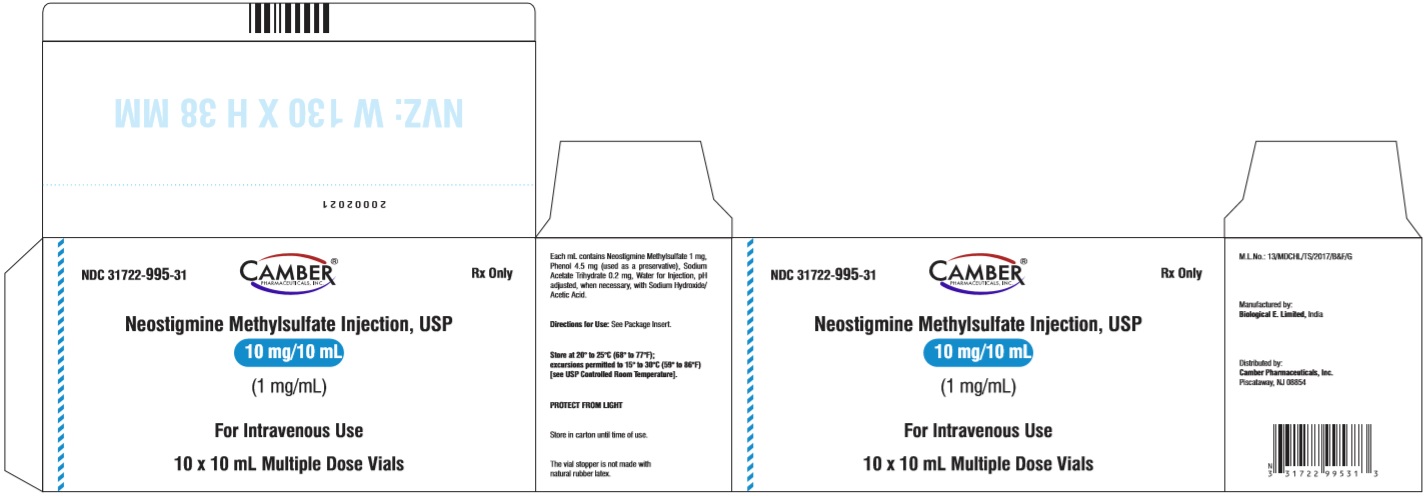
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNeostigmine Methylsulfate Injection USP 5 mg/10 mL (0.5 mg/mL) - Vial Label - Neostigmine Methylsulfate Injection USP 5 mg/10 mL (0.5 mg/mL) - Carton Label - Neostigmine Methylsulfate ...
-
INGREDIENTS AND APPEARANCEProduct Information