Label: GLOSTRIPS- fluorescein sodium strip
- NDC Code(s): 51801-009-40, 51801-009-50
- Packager: Nomax Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 23, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFluorescein Sodium Ophthalmic Strips U.S.P. diagnostic agent is for professional use only - Each strip is impregnated with 1.0 mg of fluorescein sodium USP.
-
INDICATIONSFor staining the anterior segment of the eye in disclosing corneal injury, in applanation tonometry and when fitting contact lenses.
-
DIRECTIONS FOR USETo ensure full fluorescence and patient comfort, the GloStrip® impregnated tip should be moistened with one or two drops of sterile, isotonic saline or irrigating solution before ...
-
HOW SUPPLIEDCarton containing 100 or 300 sterile strips.
-
SPL UNCLASSIFIED SECTIONNomax, Inc. • St. Louis, MO 63123 USA - Rev. 07/13 - MSN 015-244
-
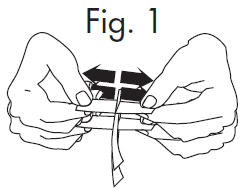
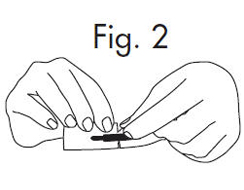
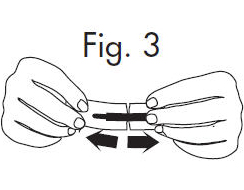
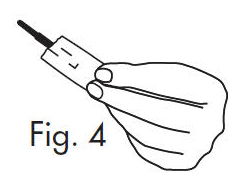
INSTRUCTIONS FOR OPENING STERILE GLOSTRIPS®
1.Grasp free tab ends of wrapping and slowly pull apart. When the white paper handle becomes visible, remove the GloStrip® from the envelope. An Alternate Method of ...
-
PRINCIPAL DISPLAY PANEL - 300 Strip CartonNDC 51801-009-50 - AMCON® Laboratories, Inc. Fluorescein - GloStrips®1.0 - 1.0 - 1.0 mg Fluorescein Sodium - Ophthalmic Strips USP - 300 Sterile Strips
-
INGREDIENTS AND APPEARANCEProduct Information