Label: ROPIVACAINE HYDROCHLORIDE injection, solution
- NDC Code(s): 71288-732-10, 71288-732-11, 71288-733-20, 71288-733-21, view more
- Packager: Meitheal Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ROPIVACAINE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for ROPIVACAINE HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGERopivacaine Hydrochloride Injection is indicated for the production of local or regional anesthesia for surgery and for acute pain management. Surgical Anesthesia: epidural block for surgery ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - There have been adverse event reports of chondrolysis in patients receiving intra-articular infusions of local anesthetics following arthroscopic and ...
-
3 DOSAGE FORMS AND STRENGTHSRopivacaine Hydrochloride Injection, USP is a clear, colorless, preservative-free solution available as: Ropivacaine Hydrochloride Injection, USP Single-Dose Vials - 0.2%, 20 mg per 10 mL (2 mg ...
-
4 CONTRAINDICATIONSRopivacaine hydrochloride injection is contraindicated in patients with a known hypersensitivity to ropivacaine or to any local anesthetic agent of the amide type.
-
5 WARNINGS AND PRECAUTIONS5.1 General Warnings and Precautions - Prior to receiving major blocks the general condition of the patient should be optimized and the patient should have an IV line inserted. All necessary ...
-
6 ADVERSE REACTIONSReactions to ropivacaine are characteristic of those associated with other amide-type local anesthetics. A major cause of adverse reactions to this group of drugs may be associated with excessive ...
-
7 DRUG INTERACTIONSPatients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available human data on use of ropivacaine hydrochloride injection in pregnant women to evaluate a drug-associated risk of major birth defects ...
-
10 OVERDOSAGEAcute emergencies from local anesthetics are generally related to high plasma levels encountered, or large doses administered, during therapeutic use of local anesthetics or to unintended ...
-
11 DESCRIPTIONRopivacaine Hydrochloride Injection, USP is a sterile, isotonic solution that contains ropivacaine hydrochloride as the active pharmaceutical ingredient. Ropivacaine hydrochloride is a member of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ropivacaine is a member of the amino amide class of local anesthetics and is supplied as the pure S-(-)-enantiomer. Local anesthetics block the generation and the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies in animals to evaluate the carcinogenic potential of ropivacaine have not been ...
-
14 CLINICAL STUDIESRopivacaine was studied as a local anesthetic both for surgical anesthesia and for acute pain management [see Dosage and Administration (2)]. The onset, depth and duration of sensory block are ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRopivacaine Hydrochloride Injection, USP is a clear, colorless, and preservative-free solution and is supplied as follows: Ropivacaine Hydrochloride Injection, USP Single-Dose Vials - NDC ...
-
17 PATIENT COUNSELING INFORMATION17.1 Information for Patients and Caregivers - When appropriate, patients should be informed in advance that they may experience temporary loss of sensation and motor activity in the anesthetized ...
-
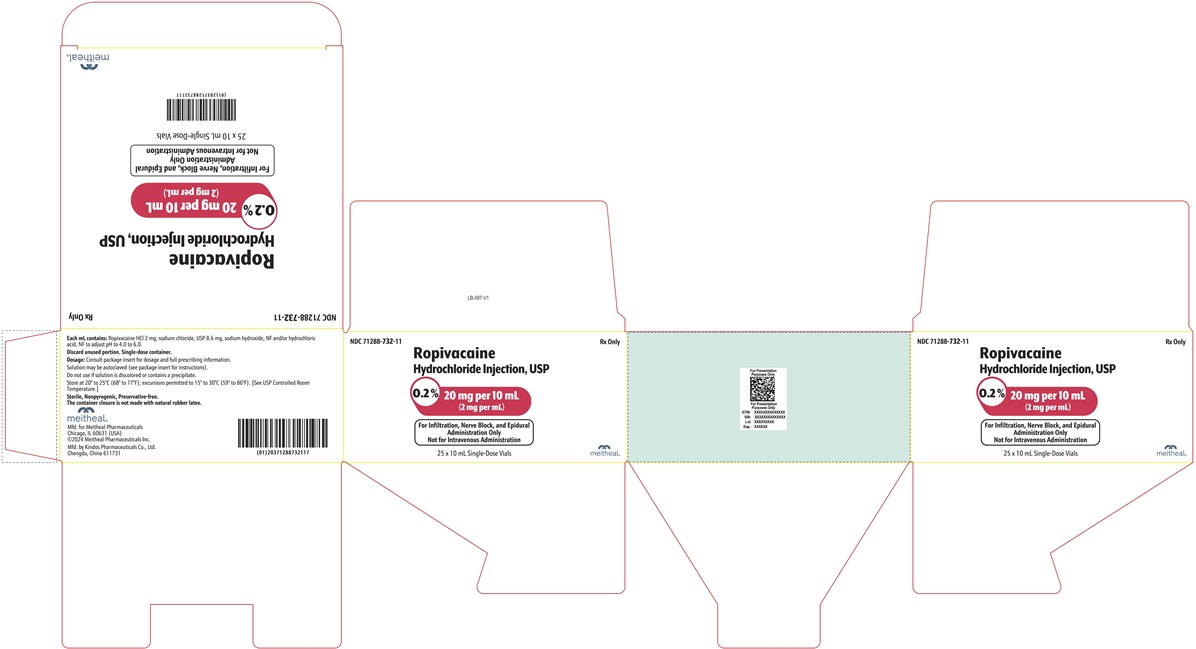
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 20 mg per 10 mL Vial LabelNDC 71288-732-10 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 0.2% 20 mg per 10 mL - (2 mg per mL) For Infiltration, Nerve Block, and Epidural Administration Only - Not for Intravenous ...
-
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 20 mg per 10 mL CartonNDC 71288-732-11 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 0.2% 20 mg per 10 mL - (2 mg per mL) For Infiltration, Nerve Block, and Epidural Administration Only - Not for Intravenous ...
-
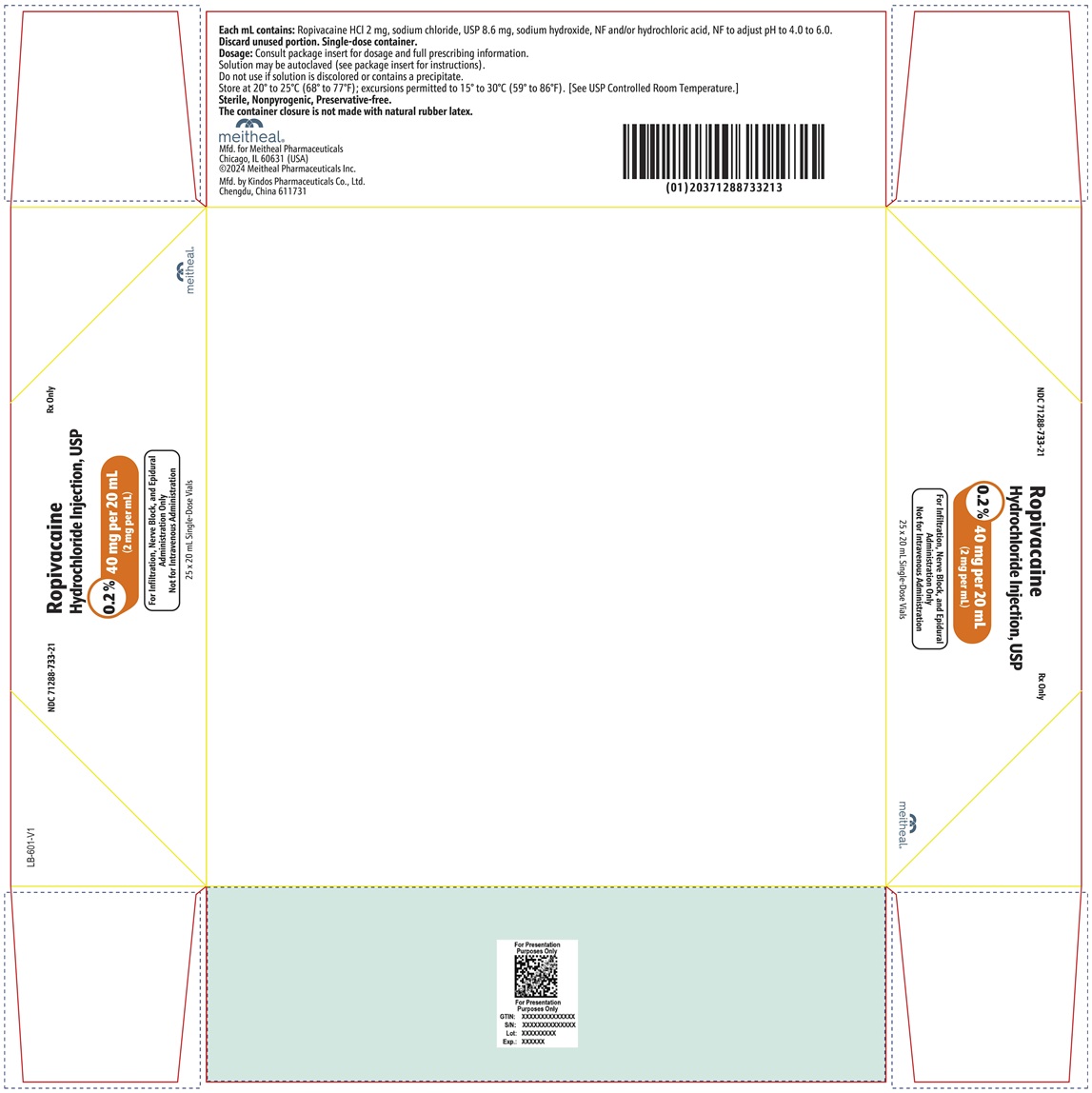
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 40 mg per 20 mL Vial LabelNDC 71288-733-20 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 0.2% 40 mg per 20 mL - (2 mg per mL) For Infiltration, Nerve Block, and Epidural Administration Only - Not for Intravenous ...
-
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 40 mg per 20 mL CartonNDC 71288-733-21 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 0.2% 40 mg per 20 mL - (2 mg per mL) For Infiltration, Nerve Block, and Epidural Administration Only - Not for Intravenous ...
-
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 100 mg per 20 mL Vial LabelNDC 71288-734-20 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 0.5% 100 mg per 20 mL - (5 mg per mL) For Infiltration, Nerve Block, and Epidural Administration Only - Not for Intravenous ...
-
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 100 mg per 20 mL CartonNDC 71288-734-21 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 0.5% 100 mg per 20 mL - (5 mg per mL) For Infiltration, Nerve Block, and Epidural Administration Only - Not for Intravenous ...
-
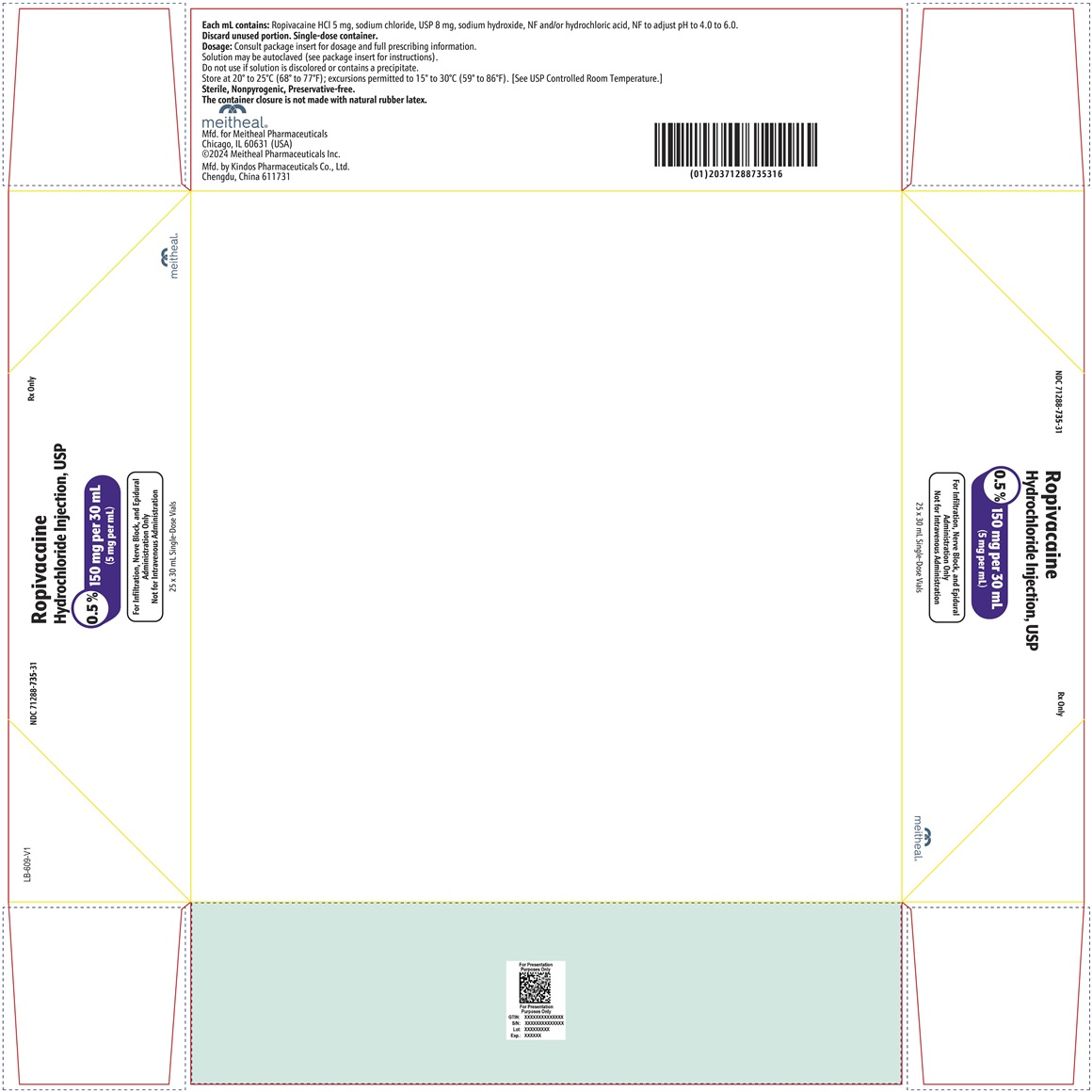
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 150 mg per 30 mL Vial LabelNDC 71288-735-30 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 0.5% 150 mg per 30 mL - (5 mg per mL) For Infiltration, Nerve Block, and Epidural Administration Only - Not for Intravenous ...
-
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 150 mg per 30 mL CartonNDC 71288-735-31 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 0.5% 150 mg per 30 mL - (5 mg per mL) For Infiltration, Nerve Block, and Epidural Administration Only - Not for Intravenous ...
-
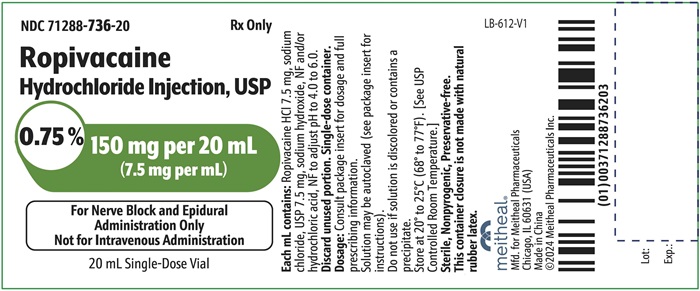
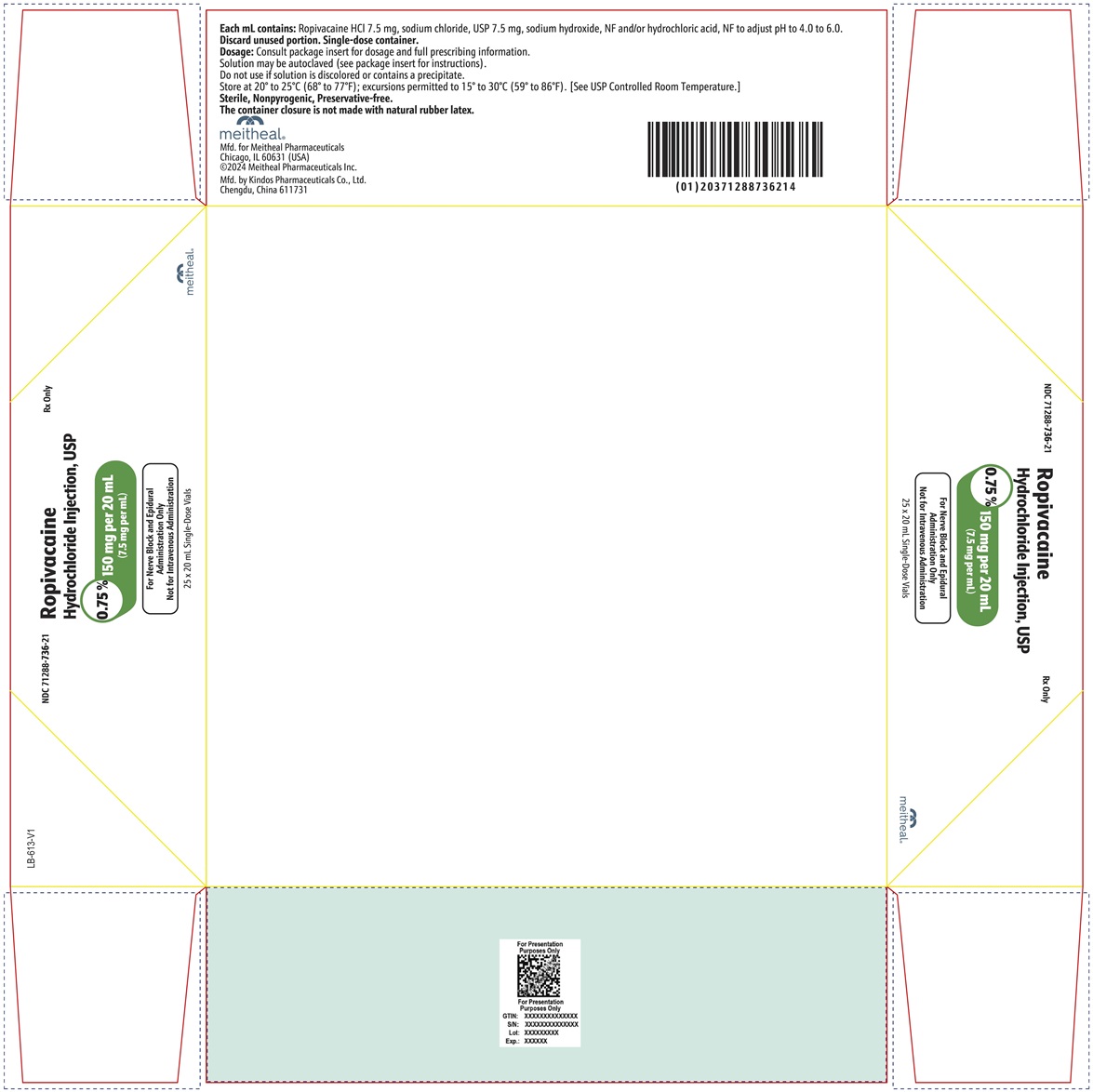
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 150 mg per 20 mL Vial LabelNDC 71288-736-20 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 0.75% 150 mg per 20 mL - (7.5 mg per mL) For Nerve Block and Epidural Administration Only - Not for Intravenous ...
-
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 150 mg per 20 mL CartonNDC 71288-736-21 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 0.75% 150 mg per 20 mL - (7.5 mg per mL) For Nerve Block and Epidural Administration Only - Not for Intravenous ...
-
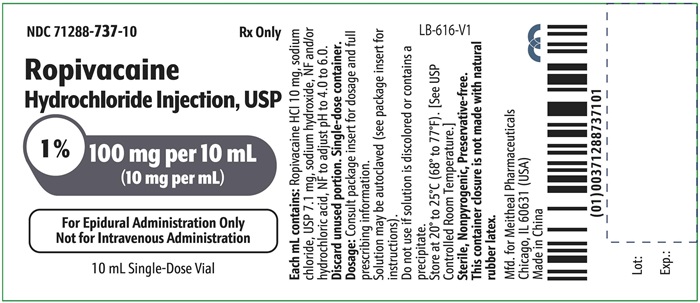
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 100 mg per 10 mL Vial LabelNDC 71288-737-10 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 1% 100 mg per 10 mL - (10 mg per mL) For Epidural Administration Only - Not for Intravenous Administration - 10 mL Single-Dose ...
-
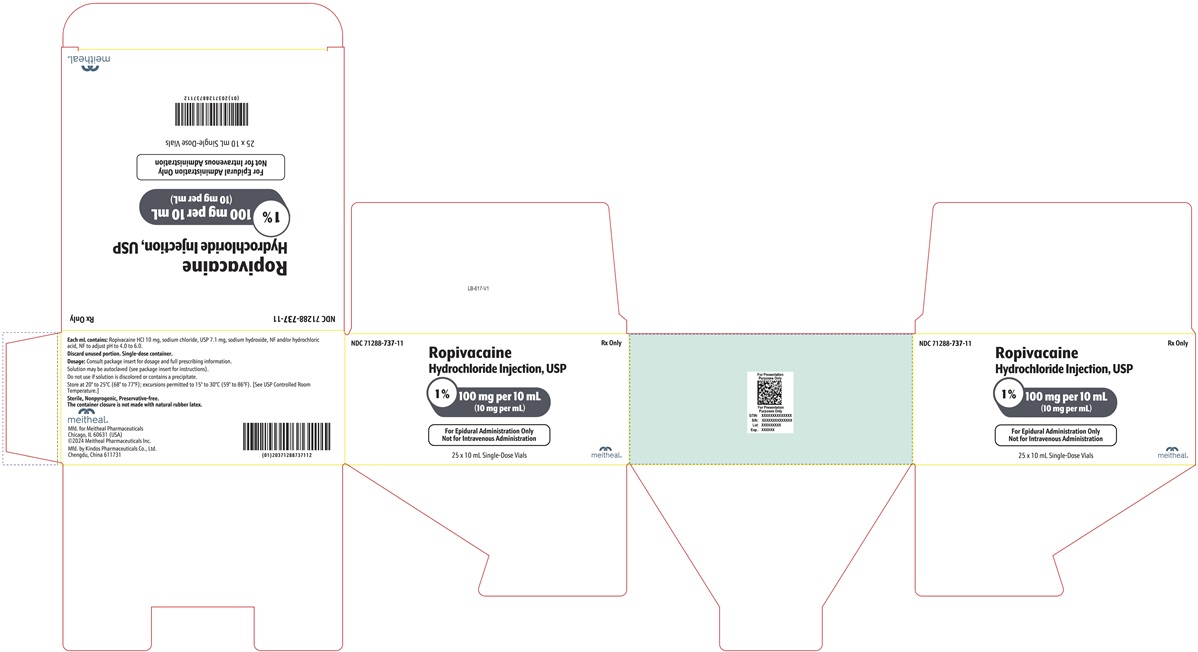
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 100 mg per 10 mL CartonNDC 71288-737-11 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 1% 100 mg per 10 mL - (10 mg per mL) For Epidural Administration Only - Not for Intravenous Administration - 25 x 10 mL ...
-
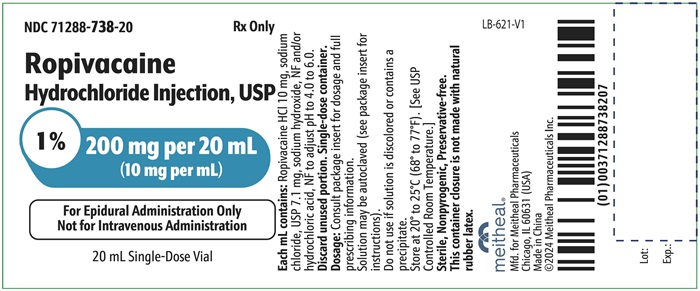
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 200 mg per 20 mL Vial LabelNDC 71288-738-20 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 1% 200 mg per 20 mL - (10 mg per mL) For Epidural Administration Only - Not for Intravenous Administration - 20 mL Single-Dose ...
-
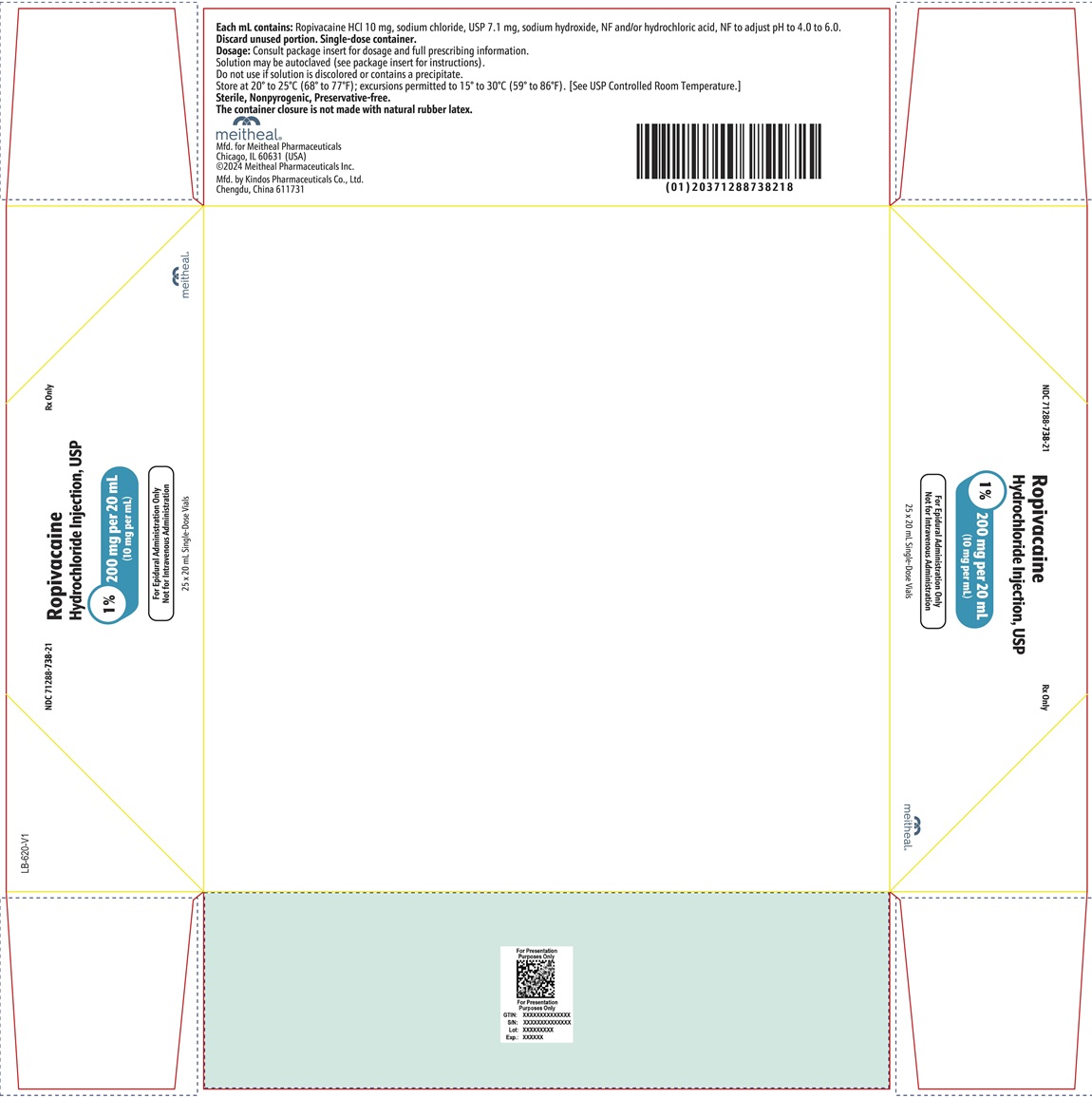
PRINCIPAL DISPLAY PANEL – Ropivacaine Hydrochloride Injection, USP 200 mg per 20 mL CartonNDC 71288-738-21 - Rx Only - Ropivacaine Hydrochloride Injection, USP - 1% 200 mg per 20 mL - (10 mg per mL) For Epidural Administration Only - Not for Intravenous Administration - 25 x 20 mL ...
-
INGREDIENTS AND APPEARANCEProduct Information