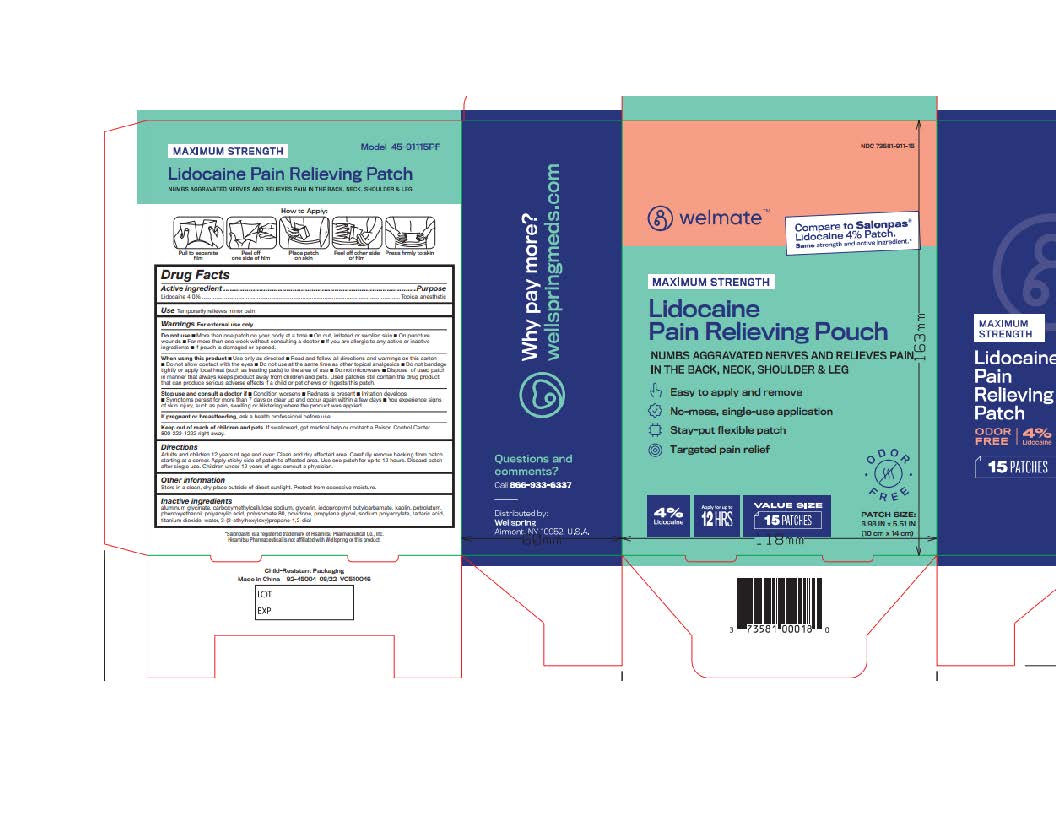
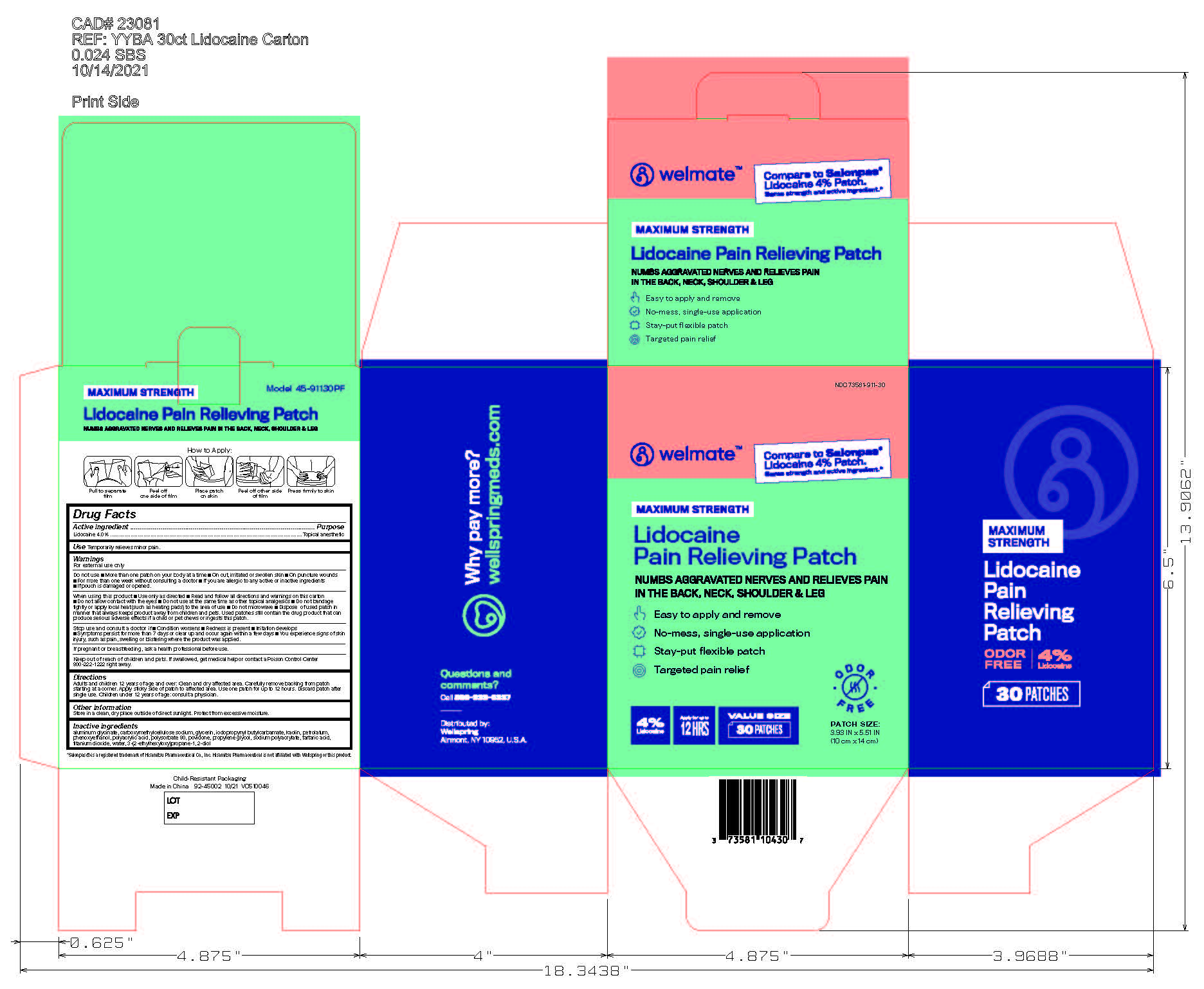
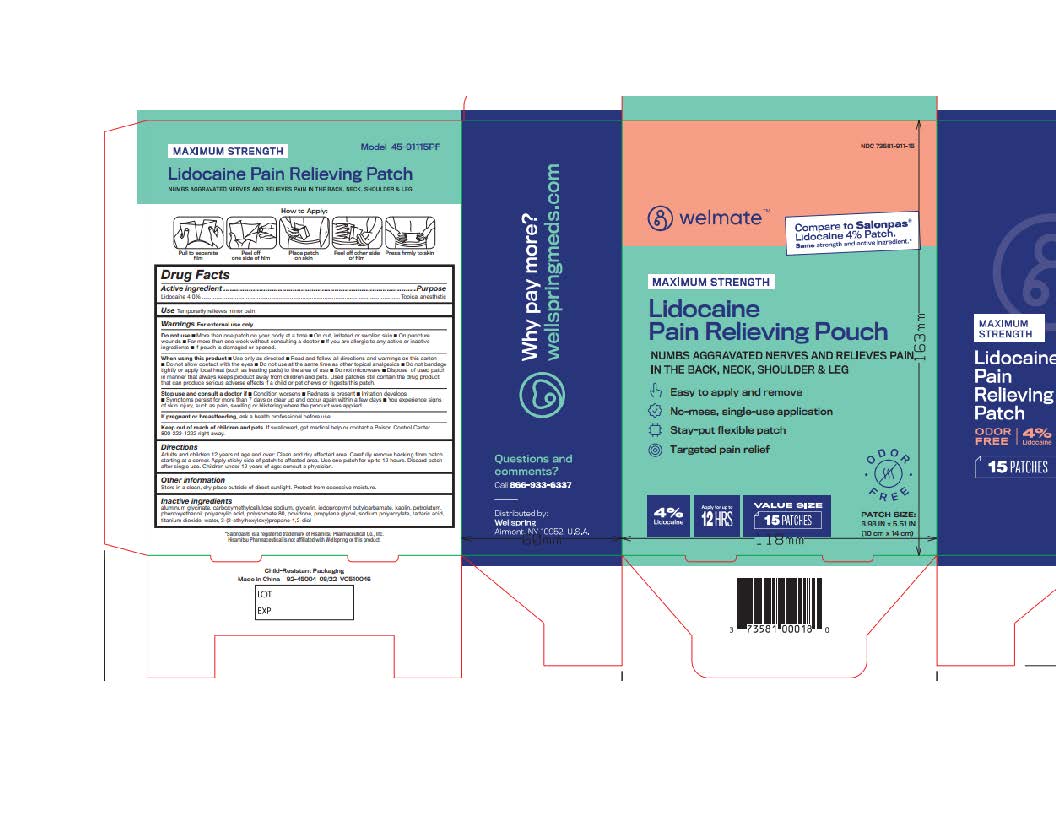
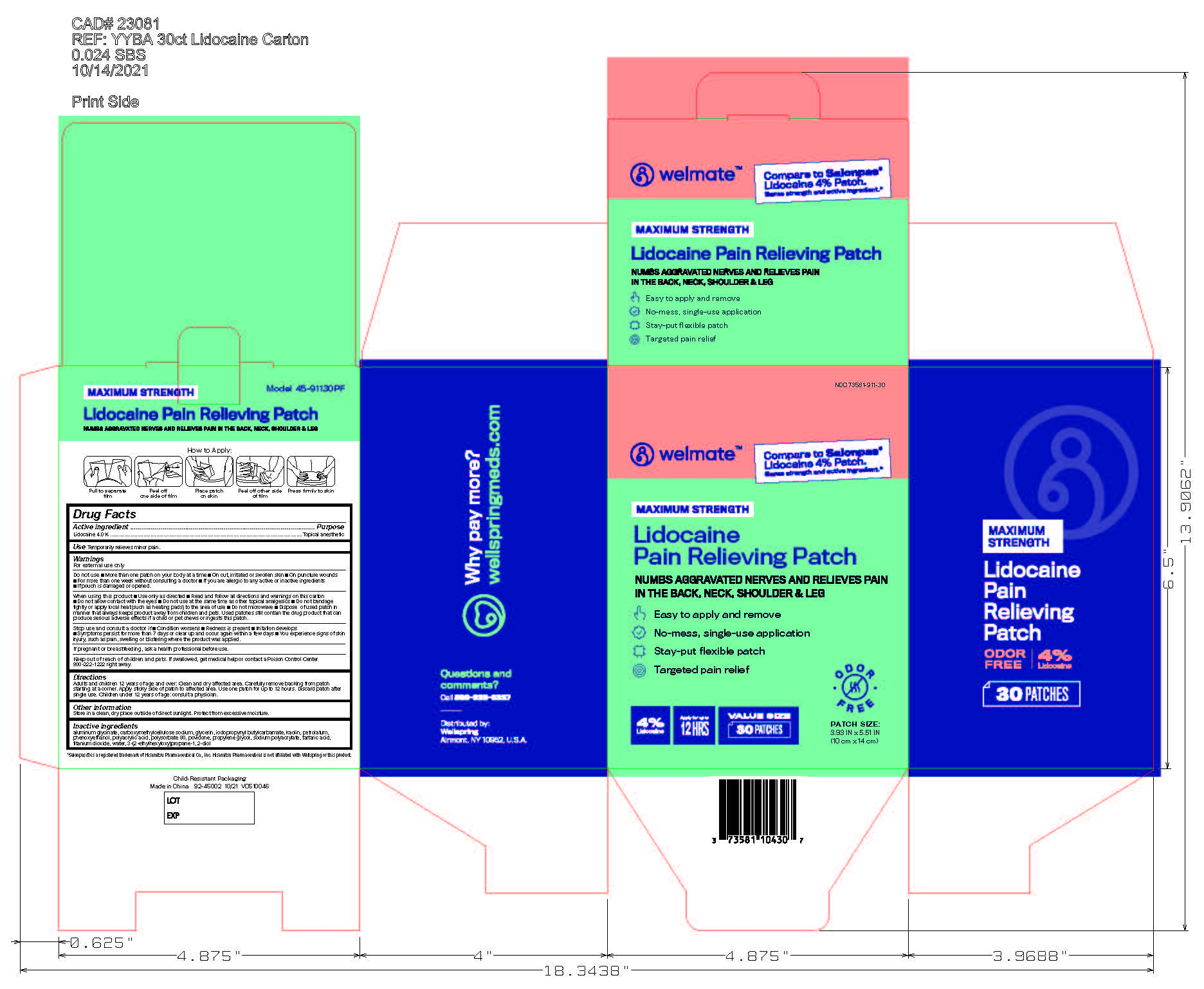
Label: WELMATE LIDOCAINE PAIN RELIEVING PATCH- lidocaine patch
- NDC Code(s): 73581-911-15, 73581-911-30
- Packager: Yyba Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- more than one patch on your body at a time
- On cut, irritated or swollen skin
- On puncture wounds
- For more than one week without consulting a doctor
- If you are allergic to any active or inactive ingredients
- if pouch is damaged or opened.
When using this product
- use only as directed
- read and follow directions and warnings on this carton
- do not allow contact with eyes.
- Do not use at the same time as other topical analgesics
- Do not bandage tightly or apply local heat (such as heating pads) to the area of use
- Do not microwave
- Dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
WELMATE LIDOCAINE PAIN RELIEVING PATCH
lidocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73581-911 Route of Administration TOPICAL, PERCUTANEOUS, TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength POLYACRYLIC ACID (8000 MW) (UNII: 73861X4K5F) PETROLATUM (UNII: 4T6H12BN9U) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) KAOLIN (UNII: 24H4NWX5CO) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73581-911-30 30 in 1 CARTON 11/01/2021 1 11 g in 1 POUCH; Type 0: Not a Combination Product 2 NDC:73581-911-15 15 in 1 CARTON 09/01/2022 2 11 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/01/2021 Labeler - Yyba Corporation (006339772) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co., Ltd., 529128763 manufacture(73581-911)