Label: LEVETIRACETAM injection
- NDC Code(s): 43547-454-10, 43547-454-41
- Packager: Solco Healthcare US, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 26, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LEVETIRACETAM INJECTION safely and effectively. See full prescribing information for LEVETIRACETAM INJECTION. LEVETIRACETAM ...These highlights do not include all the information needed to use LEVETIRACETAM INJECTION safely and effectively. See full prescribing information for LEVETIRACETAM INJECTION.
LEVETIRACETAM injection, for intravenous use
Initial U.S. Approval: 1999RECENT MAJOR CHANGES
Warnings and Precautions (5.5) 3/2024
INDICATIONS AND USAGE
- •
- Levetiracetam injection is indicated for the treatment of partial-onset seizures in patients 1 month of age and older (1.1)
- •
- Levetiracetam injection is indicated for adjunctive therapy for the treatment of:
- o
- Myoclonic seizures in patients 12 years of age and older with juvenile myoclonic epilepsy (1.2)
- o
- Primary generalized tonic-clonic seizures in patients 6 years of age and older with idiopathic generalized epilepsy (1.3)
- •
- Levetiracetam injection is for intravenous use only as an alternative for patients when oral administration is temporarily not feasible (1.4)
DOSAGE AND ADMINISTRATION
Levetiracetam injection is for intravenous use only (2.1)
Partial-Onset Seizures (monotherapy or adjunctive therapy)
- •
- 1 Month to < 6 Months: 7 mg/kg twice daily; increase by 7 mg/kg twice daily every 2 weeks to recommended dose of 21 mg/kg twice daily (2.1)
- •
- 6 Months to < 4 Years: 10 mg/kg twice daily; increase by 10 mg/kg twice daily every 2 weeks to recommended dose of 25 mg/kg twice daily (2.1)
- •
- 4 Years to < 16 Years: 10 mg/kg twice daily; increase by 10 mg/kg twice daily every 2 weeks to recommended dose of 30 mg/kg twice daily (2.1)
- •
- Adults 16 Years and Older: 500 mg twice daily; increase by 500 mg twice daily every 2 weeks to a recommended dose of 1,500 mg twice daily (2.1)
Myoclonic Seizures in Adults and Pediatric Patients 12 Years and Older
- •
- 500 mg twice daily; increase by 500 mg twice daily every 2 weeks to recommended dose of 1,500 mg twice daily (2.2)
Primary Generalized Tonic-Clonic Seizures
- •
- 6 Years to < 16 Years: 10 mg/kg twice daily; increase by 10 mg/kg twice daily every 2 weeks to recommended dose of 30 mg/kg twice daily (2.3)
- •
- Adults 16 Years and Older: 500 mg twice daily; increase by 500 mg twice daily every 2 weeks to recommended dose of 1,500 mg twice daily (2.3)
Switching From or To Oral Levetiracetam
When switching from or to oral levetiracetam, the total daily dosage/frequency of levetiracetam injection should be equivalent to those of oral levetiracetam (2.4, 2.5)
See full prescribing information for preparation and administration instructions (2.6) and dosage adjustment in adults with renal impairment (2.7)
DOSAGE FORMS AND STRENGTHS
Injection: 500 mg/5 mL single-dose vial (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Behavioral abnormalities including psychotic symptoms, suicidal ideation, irritability, and aggressive behavior have been observed; monitor patients for psychiatric signs and symptoms (5.1)
- •
- Monitor for somnolence and fatigue; advise patients not to drive or operate machinery until they have sufficient experience on levetiracetam (5.2)
- •
- Serious Dermatological Reactions: Discontinue levetiracetam at the first sign of rash unless clearly not drug related (5.4)
- •
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity: Discontinue if no alternative etiology (5.5)
- •
- Coordination Difficulties: Monitor for ataxia, abnormal gait, and incoordination (5.6)
- •
- Withdrawal Seizures: Levetiracetam must be gradually withdrawn (5.7)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5% more than placebo) include:
- •
- Adults: somnolence, asthenia, infection, and dizziness (6.1)
- •
- Pediatric patients: fatigue, aggression, nasal congestion, decreased appetite, and irritability (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Solco Healthcare US, LLC at 1-866-257-2597 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pregnancy: Plasma levels of levetiracetam may be decreased; monitor closely during pregnancy. Based on animal data, may cause fetal harm (5.10, 8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2025
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Partial-Onset Seizures
1.2 Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy
1.3 Primary Generalized Tonic-Clonic Seizures
1.4 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Dosing for Partial-Onset Seizures
2.2 Dosing for Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy
2.3 Dosing for Primary Generalized Tonic-Clonic Seizures
2.4 Switching from Oral Dosing
2.5 Switching to Oral Dosing
2.6 Preparation and Administration Instructions
2.7 Dosage Adjustments in Adult Patients with Renal Impairment
2.8 Discontinuation of Levetiracetam
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Behavioral Abnormalities and Psychotic Symptoms
5.2 Somnolence and Fatigue
5.3 Anaphylaxis and Angioedema
5.4 Serious Dermatological Reactions
5.5 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
5.6 Coordination Difficulties
5.7 Withdrawal Seizures
5.8 Hematologic Abnormalities
5.9 Increase in Blood Pressure
5.10 Seizure Control During Pregnancy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
10.1 Signs, Symptoms and Laboratory Findings of Acute Overdosage in Humans
10.2 Management of Overdose
10.3 Hemodialysis
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Partial-Onset Seizures
14.2 Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy
14.3 Primary Generalized Tonic-Clonic Seizures
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE 1.1 Partial-Onset Seizures - Levetiracetam injection is indicated for the treatment of partial-onset seizures in patients 1 month of age and older. 1.2 Myoclonic Seizures in Patients with ...
1.1 Partial-Onset Seizures
Levetiracetam injection is indicated for the treatment of partial-onset seizures in patients 1 month of age and older.
1.2 Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy
Levetiracetam injection is indicated as adjunctive therapy for the treatment of myoclonic seizures in patients 12 years of age and older with juvenile myoclonic epilepsy.
1.3 Primary Generalized Tonic-Clonic Seizures
Levetiracetam injection is indicated as adjunctive therapy for the treatment of primary generalized tonic-clonic seizures in patients 6 years of age and older with idiopathic generalized epilepsy.
Close1.4 Limitations of Use
Levetiracetam injection is for intravenous use only as an alternative for patients when oral administration is temporarily not feasible.
-
2 DOSAGE AND ADMINISTRATION 2.1 Dosing for Partial-Onset Seizures - The recommended dosing for monotherapy and adjunctive therapy is the same as outlined below. There is no clinical study experience with administration of ...
2.1 Dosing for Partial-Onset Seizures
The recommended dosing for monotherapy and adjunctive therapy is the same as outlined below.
There is no clinical study experience with administration of intravenous levetiracetam for a period longer than 4 days.
Adults 16 Years of Age and Older
Initiate treatment with a daily dose of 1,000 mg/day, given as twice-daily dosing (500 mg twice daily). Additional dosing increments may be given (1,000 mg/day additional every 2 weeks) to a maximum recommended daily dose of 3,000 mg. There is no evidence that doses greater than 3,000 mg/day confer additional benefit.
Pediatric Patients
1 Month to < 6 Months
- Initiate treatment with a daily dose of 14 mg/kg in 2 divided doses (7 mg/kg twice daily). Increase the daily dose every 2 weeks by increments of 14 mg/kg to the recommended daily dose of 42 mg/kg (21 mg/kg twice daily). In the clinical trial, the mean daily dose was 35 mg/kg in this age group.
6 Months to < 4 Years
- Initiate treatment with a daily dose of 20 mg/kg in 2 divided doses (10 mg/kg twice daily). Increase the daily dose in 2 weeks by an increment of 20 mg/kg to the recommended daily dose of 50 mg/kg (25 mg/kg twice daily). If a patient cannot tolerate a daily dose of 50 mg/kg, the daily dose may be reduced. In the clinical trial, the mean daily dose was 47 mg/kg in this age group.
4 Years to < 16 Years
- Initiate treatment with a daily dose of 20 mg/kg in 2 divided doses (10 mg/kg twice daily). Increase the daily dose every 2 weeks by increments of 20 mg/kg to the recommended daily dose of 60 mg/kg (30 mg/kg twice daily). If a patient cannot tolerate a daily dose of 60 mg/kg, the daily dose may be reduced. In the clinical trial, the mean daily dose was 44 mg/kg. The maximum daily dose was 3,000 mg/day.
2.2 Dosing for Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy
Initiate treatment with a dose of 1,000 mg/day, given as twice-daily dosing (500 mg twice daily). Increase the dosage by 1,000 mg/day every 2 weeks to the recommended daily dose of 3,000 mg. The effectiveness of doses lower than 3,000 mg/day has not been studied.
2.3 Dosing for Primary Generalized Tonic-Clonic Seizures
Adults 16 Years of Age and Older
Initiate treatment with a dose of 1,000 mg/day, given as twice-daily dosing (500 mg twice daily). Increase dosage by 1,000 mg/day every 2 weeks to the recommended daily dose of 3,000 mg. The effectiveness of doses lower than 3,000 mg/day has not been adequately studied.
Pediatric Patients 6 to <16 Years of Age
Initiate treatment with a daily dose of 20 mg/kg in 2 divided doses (10 mg/kg twice daily). Increase the daily dose every 2 weeks by increments of 20 mg/kg (10 mg/kg twice daily) to the recommended daily dose of 60 mg/kg (30 mg/kg twice daily). The effectiveness of doses lower than 60 mg/kg/day has not been adequately studied.
2.4 Switching from Oral Dosing
When switching from oral levetiracetam, the initial total daily intravenous dosage of levetiracetam should be equivalent to the total daily dosage and frequency of oral levetiracetam.
2.5 Switching to Oral Dosing
At the end of the intravenous treatment period, the patient may be switched to levetiracetam oral administration at the equivalent daily dosage and frequency of the intravenous administration.
2.6 Preparation and Administration Instructions
Levetiracetam injection is for intravenous use only and should be diluted in 100 mL of a compatible diluent prior to administration. If a smaller volume is required (e.g. pediatric patients), the amount of diluent should be calculated to not exceed a maximum levetiracetam concentration of 15 mg per mL of diluted solution. Consideration should also be given to the total daily fluid intake of the patient. Levetiracetam injection should be administered as a 15-minute intravenous infusion. One vial of levetiracetam injection contains 500 mg levetiracetam (500 mg/5 mL).
Levetiracetam injection may be mixed with the following diluents and antiepileptic drugs and may be stored in polyvinyl chloride (PVC) bags. The diluted solution should not be stored for more than 4 hours at controlled room temperature [15-30oC (59-86oF)].
Diluents:
Sodium chloride (0.9%) injection, USP
Lactated Ringer’s injection
Dextrose 5% injection, USP
Other Antiepileptic Drugs:
Lorazepam
Diazepam
Valproate sodium
There are no data to support the physical compatibility of levetiracetam injection with antiepileptic drugs that are not listed above.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Product with particulate matter or discoloration should not be used.
Any unused portion of the levetiracetam injection vial contents should be discarded.
Adults
See Table 1 for the recommended preparation and administration of levetiracetam injection for adults to achieve a dose of 500 mg, 1,000 mg, or 1,500 mg.
Table 1: Preparation and Administration of Levetiracetam Injection for Adults Dose
Withdraw Volume
Volume of Diluent
Infusion Time
500 mg
5 mL (5 mL vial)
100 mL
15 minutes
1,000 mg
10 mL (two 5 mL vials)
100 mL
15 minutes
1,500 mg
15 mL (three 5 mL vials)
100 mL
15 minutes
For example, to prepare a 1,000 mg dose, dilute 10 mL of levetiracetam injection in 100 mL of a compatible diluent and administer intravenously as a 15-minute infusion.
Pediatric Patients
When using levetiracetam injection for pediatric patients, dosing is weight-based (mg per kg).
The following calculation should be used to determine the appropriate daily dose of levetiracetam injection for pediatric patients:
2.7 Dosage Adjustments in Adult Patients with Renal Impairment
Levetiracetam dosing must be individualized according to the patient's renal function status. Recommended dosage adjustments for adults with renal impairment are shown in Table 2. Information is unavailable for dosage adjustments in pediatric patients with renal impairment. In order to calculate the dose recommended for adult patients with renal impairment, creatinine clearance adjusted for body surface area must be calculated. To do this an estimate of the patient’s creatinine clearance (CLcr) in mL/min must first be calculated using the following formula:
Then CLcr is adjusted for body surface area (BSA) as follows:
Table 2: Dosage Adjustment Regimen for Adult Patients with Renal Impairment Group
Creatinine Clearance (mL/min/1.73m2)
Dosage (mg)
Frequency
Normal
> 80
500 to 1,500
Every 12 hours
Mild
50 – 80
500 to 1,000
Every 12 hours
Moderate
30 – 50
250 to 750
Every 12 hours
Severe
< 30
250 to 500
Every 12 hours
ESRD patients using dialysis
------
500 to 1,0001
Every 24 hours1
1 Following dialysis, a 250 to 500 mg supplemental dose is recommended.
Close2.8 Discontinuation of Levetiracetam
Avoid abrupt withdrawal from levetiracetam in order to reduce the risk of increased seizure frequency and status epilepticus [see Warnings and Precautions (5.7)].
-
3 DOSAGE FORMS AND STRENGTHS One vial of levetiracetam injection, USP, contains 500 mg levetiracetam, USP, (500 mg/5 mL) as a clear, colorless solution.
One vial of levetiracetam injection, USP, contains 500 mg levetiracetam, USP, (500 mg/5 mL) as a clear, colorless solution.
Close -
4 CONTRAINDICATIONS Levetiracetam injection is contraindicated in patients with a hypersensitivity to levetiracetam. Reactions have included anaphylaxis and angioedema [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS 5.1 Behavioral Abnormalities and Psychotic Symptoms - Levetiracetam may cause behavioral abnormalities and psychotic symptoms. Patients treated with levetiracetam should be monitored for ...
5.1 Behavioral Abnormalities and Psychotic Symptoms
Levetiracetam may cause behavioral abnormalities and psychotic symptoms. Patients treated with levetiracetam should be monitored for psychiatric signs and symptoms.
Behavioral abnormalities
In clinical studies using an oral formulation of levetiracetam, 13% of adult levetiracetam-treated patients and 38% of pediatric levetiracetam-treated patients (4 to 16 years of age), compared to 6% and 19% of adult and pediatric placebo-treated patients, experienced non-psychotic behavioral symptoms (reported as aggression, agitation, anger, anxiety, apathy, depersonalization, depression, emotional lability, hostility, hyperkinesias, irritability, nervousness, neurosis, and personality disorder).
A randomized, double-blind, placebo-controlled study was performed to assess the neurocognitive and behavioral effects of an oral formulation of levetiracetam as adjunctive therapy in pediatric patients (4 to 16 years of age). The results from an exploratory analysis indicated a worsening in levetiracetam-treated patients on aggressive behavior (one of eight behavior dimensions), as measured in a standardized and systematic way using a validated instrument, the Achenbach Child Behavior Checklist (CBCL/6-18).
In clinical studies in pediatric patients 1 month to < 4 years of age, irritability was reported in 12% of the levetiracetam-treated patients compared to 0% of placebo-treated patients.
In clinical studies, 1.7% of adult levetiracetam-treated patients discontinued treatment due to behavioral adverse reactions, compared to 0.2% of placebo-treated patients. The treatment dose was reduced in 0.8% of adult levetiracetam-treated patients and in 0.5% of placebo-treated patients. Overall, 11% of levetiracetam-treated pediatric patients experienced behavioral symptoms associated with discontinuation or dose reduction, compared to 6% of placebo-treated patients.
Psychotic symptoms
In clinical studies using an oral formulation of levetiracetam, 1% of levetiracetam-treated adult patients, 2% of levetiracetam-treated pediatric patients 4 to 16 years of age, and 17% of levetiracetam-treated pediatric patients 1 month to <4 years of age experienced psychotic symptoms, compared to 0.2%, 2%, and 5% in the corresponding age groups treated with placebo. In a controlled study that assessed the neurocognitive and behavioral effects of an oral formulation of levetiracetam in pediatric patients 4 to 16 years of age, 1.6% of levetiracetam-treated patients experienced paranoia, compared to 0% of placebo-treated patients. In the same study, 3.1% of levetiracetam-treated patients experienced confusional state, compared to 0% of placebo-treated patients [see Use in Specific Populations (8.4)].
In clinical studies, two (0.3%) levetiracetam-treated adult patients were hospitalized, and their treatment was discontinued due to psychosis. Both events, reported as psychosis, developed within the first week of treatment and resolved within 1 to 2 weeks following treatment discontinuation. There was no difference between drug- and placebo-treated patients in the incidence of the pediatric patients who discontinued treatment due to psychotic and non-psychotic adverse reactions.
5.2 Somnolence and Fatigue
Levetiracetam may cause somnolence and fatigue. Patients should be monitored for somnolence and fatigue, and be advised not to drive or operate machinery until they have gained sufficient experience on levetiracetam to gauge whether it adversely affects their ability to drive or operate machinery.
Somnolence
In controlled clinical studies using an oral formulation of levetiracetam in adult patients with partial-onset seizures, 15% of levetiracetam-treated patients reported somnolence, compared to 8% of placebo-treated patients. There was no clear dose response up to 3,000 mg/day. In a study in which there was no titration, about 45% of patients receiving levetiracetam 4,000 mg/day reported somnolence. The somnolence was considered serious in 0.3% of levetiracetam-treated patients, compared to 0% in the placebo group. About 3% of levetiracetam-treated patients discontinued treatment due to somnolence, compared to 0.7% of placebo-treated patients. In 1.4% of levetiracetam-treated patients and 0.9% of placebo-treated patients, the dose was reduced, while 0.3% of the levetiracetam-treated patients were hospitalized due to somnolence.
Asthenia
In controlled clinical studies using an oral formulation of levetiracetam in adult patients with partial-onset seizures, 15% of levetiracetam-treated patients reported asthenia, compared to 9% of placebo-treated patients. Treatment was discontinued due to asthenia in 0.8% of levetiracetam-treated patients as compared to 0.5% of placebo-treated patients. In 0.5% of levetiracetam-treated patients and in 0.2% of placebo-treated patients, the dose was reduced due to asthenia.
Somnolence and asthenia occurred most frequently within the first 4 weeks of treatment. In general, the incidences of somnolence and fatigue in the pediatric partial-onset seizure studies, and in pediatric and adult myoclonic and primary generalized tonic-clonic studies were comparable to those of the adult partial-onset seizure studies.
5.3 Anaphylaxis and Angioedema
Levetiracetam injection can cause anaphylaxis or angioedema after the first dose or at any time during treatment. Signs and symptoms in cases reported in the postmarketing setting have included hypotension, hives, rash, respiratory distress, and swelling of the face, lip, mouth, eye, tongue, throat, and feet. In some reported cases, reactions were life-threatening and required emergency treatment. If a patient develops signs or symptoms of anaphylaxis or angioedema, levetiracetam injection should be discontinued and the patient should seek immediate medical attention. Levetiracetam injection should be discontinued permanently if a clear alternative etiology for the reaction cannot be established [see Contraindications (4)].
5.4 Serious Dermatological Reactions
Serious dermatological reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported in both pediatric and adult patients treated with levetiracetam. The median time of onset is reported to be 14 to 17 days, but cases have been reported at least four months after initiation of treatment. Recurrence of the serious skin reactions following rechallenge with levetiracetam has also been reported. Levetiracetam should be discontinued at the first sign of a rash, unless the rash is clearly not drug-related. If signs or symptoms suggest SJS/TEN, use of this drug should not be resumed and alternative therapy should be considered.
5.5 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan hypersensitivity, has been reported in patients taking antiepileptic drugs, including levetiracetam. These events can be fatal or life-threatening, particularly if diagnosis and treatment do not occur as early as possible. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling, in association with other organ system involvement, such as hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis, sometimes resembling an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its expression, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. Levetiracetam should be discontinued if an alternative etiology for the signs or symptoms cannot be established [see Contraindications (4)].
5.6 Coordination Difficulties
Levetiracetam may cause coordination difficulties.
In controlled clinical studies using an oral formulation of levetiracetam in adult patients with partial-onset seizures, 3.4% of levetiracetam-treated patients experienced coordination difficulties, (reported as ataxia, abnormal gait, or incoordination) compared to 1.6% of placebo-treated patients. A total of 0.4% of patients in controlled clinical studies discontinued levetiracetam treatment due to ataxia, compared to 0% of placebo-treated patients. In 0.7% of levetiracetam-treated patients and in 0.2% of placebo-treated patients, the dose was reduced due to coordination difficulties, while one of the treated patients was hospitalized due to worsening of pre-existing ataxia. These events occurred most frequently within the first 4 weeks of treatment.
Patients should be monitored for signs and symptoms of coordination difficulties and advised not to drive or operate machinery until they have gained sufficient experience on levetiracetam to gauge whether it could adversely affect their ability to drive or operate machinery.
5.7 Withdrawal Seizures
As with most antiepileptic drugs, levetiracetam should generally be withdrawn gradually because of the risk of increased seizure frequency and status epilepticus. But if withdrawal is needed because of a serious adverse reaction, rapid discontinuation can be considered.
5.8 Hematologic Abnormalities
Levetiracetam can cause hematologic abnormalities. Hematologic abnormalities occurred in clinical trials and included decreases in white blood cell (WBC), neutrophil, and red blood cells counts (RBC); decreases in hemoglobin and hematocrit; and increases in eosinophil counts. Cases of agranulocytosis, pancytopenia, and thrombocytopenia have been reported in the postmarketing setting. A complete blood count is recommended in patients experiencing significant weakness, pyrexia, recurrent infections, or coagulation disorders.
Partial-Onset Seizures
Adults
In controlled clinical studies using an oral formulation of levetiracetam in adult patients with partial-onset seizures, minor but statistically significant decreases compared to placebo in total mean RBC (0.03×106/mm3), mean hemoglobin (0.09 g/dL), and mean hematocrit (0.38%), were seen in levetiracetam-treated patients.
A total of 3.2% of levetiracetam-treated and 1.8% of placebo-treated patients had at least one possibly significant (≤2.8×109/L) decreased WBC, and 2.4% of levetiracetam-treated and 1.4% of placebo-treated patients had at least one possibly significant (≤1.0×109/L) decreased neutrophil count. Of the levetiracetam-treated patients with a low neutrophil count, all but one rose towards or to baseline with continued treatment. No patient was discontinued secondary to low neutrophil counts.
Pediatric Patients 4 Years to < 16 Years
In a controlled study in pediatric patients age 4 years to <16 years, statistically significant decreases in WBC and neutrophil counts were seen in levetiracetam-treated patients, as compared to placebo. The mean decreases from baseline in the levetiracetam-treated group were -0.4×109/L and -0.3×109/L, respectively, whereas there were small increases in the placebo group. Mean relative lymphocyte counts increased by 1.7% in levetiracetam-treated patients, compared to a decrease of 4% in placebo-treated patients (statistically significant).
More levetiracetam-treated patients had a possibly clinically significant abnormally low WBC value (3% of levetiracetam-treated patients versus 0% of placebo-treated patients); however, there was no apparent difference between treatment groups with respect to neutrophil count (5% on levetiracetam versus 4.2% on placebo). No patient was discontinued because of low WBC or neutrophil count.
In a randomized, double-blind, placebo-controlled study to assess the neurocognitive and behavioral effects of an oral formulation of levetiracetam as adjunctive therapy in pediatric patients (4 to 16 years of age), 5 patients (8.6%) in the levetiracetam-treated group and two patients (6.1%) in the placebo-treated group had high eosinophil count values that were possibly clinically significant (≥10% or ≥0.7×109/L).
5.9 Increase in Blood Pressure
In a randomized, placebo-controlled study in patients 1 month to <4 years of age using an oral formulation of levetiracetam, a significantly higher risk of increased diastolic blood pressure was observed in the levetiracetam-treated patients (17%), compared to placebo-treated patients (2%). There was no overall difference in mean diastolic blood pressure between the treatment groups. This disparity between the levetiracetam and placebo treatment groups was not observed in the studies of older children or in adults.
Monitor patients 1 month to <4 years of age for increases in diastolic blood pressure.
Close5.10 Seizure Control During Pregnancy
Physiological changes may gradually decrease plasma levels of levetiracetam throughout pregnancy. This decrease is more pronounced during the third trimester. It is recommended that patients be monitored carefully during pregnancy. Close monitoring should continue through the postpartum period especially if the dose was changed during pregnancy.
-
6 ADVERSE REACTIONS The following adverse reactions are discussed in more details in other sections of labeling: • Behavioral Abnormalities and Psychotic Symptoms [see Warnings and Precautions (5.1)] • Somnolence ...
The following adverse reactions are discussed in more details in other sections of labeling:
- •
- Behavioral Abnormalities and Psychotic Symptoms [see Warnings and Precautions (5.1)]
- •
- Somnolence and Fatigue [see Warnings and Precautions (5.2)]
- •
- Anaphylaxis and Angioedema [see Warnings and Precautions (5.3)]
- •
- Serious Dermatological Reactions [see Warnings and Precautions (5.4)]
- •
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity [see Warnings and Precautions (5.5)]
- •
- Coordination Difficulties [see Warnings and Precautions (5.6)]
- •
- Hematologic Abnormalities [see Warnings and Precautions (5.8)]
- •
- Increase in Blood Pressure [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse reactions that result from levetiracetam injection use include all of those reported for levetiracetam tablets and oral solution. Equivalent doses of intravenous (IV) levetiracetam and oral levetiracetam result in equivalent Cmax, Cmin, and total systemic exposure to levetiracetam when the intravenous levetiracetam is administered as a 15-minute infusion.
Partial-Onset Seizures
Adults
In controlled clinical studies using levetiracetam tablets in adults with partial-onset seizures [see Clinical Studies (14.1)], the most common adverse reactions in adult patients receiving levetiracetam in combination with other AEDs, for events with rates greater than placebo, were somnolence, asthenia, infection, and dizziness. Of the most common adverse reactions in adults experiencing partial-onset seizures, asthenia, somnolence, and dizziness occurred predominantly during the first 4 weeks of treatment with levetiracetam.
Table 3 lists adverse reactions that occurred in at least 1% of adult epilepsy patients receiving levetiracetam tablets in placebo-controlled studies and were numerically more common than in patients treated with placebo. In these studies, either levetiracetam or placebo was added to concurrent AED therapy.
Table 3: Adverse Reactions* in Pooled Placebo-Controlled, Adjunctive Studies in Adults Experiencing Partial-Onset Seizures Levetiracetam
(N=769)
%
Placebo
(N=439)
%
- Asthenia
15
9
- Somnolence
15
8
- Headache
14
13
- Infection
13
8
- Dizziness
9
4
- Pain
7
6
- Pharyngitis
6
4
- Depression
4
2
- Nervousness
4
2
- Rhinitis
4
3
- Anorexia
3
2
- Ataxia
3
1
- Vertigo
3
1
- Amnesia
2
1
- Anxiety
2
1
- Cough Increased
2
1
- Diplopia
2
1
- Emotional Lability
2
0
- Hostility
2
1
- Paresthesia
2
1
- Sinusitis
2
1
* Adverse reactions occurred in at least 1% of levetiracetam-treated patients and occurred more frequently than placebo-treated patients
In controlled adult clinical studies using levetiracetam tablets, 15% of patients receiving levetiracetam and 12% receiving placebo either discontinued or had a dose reduction as a result of an adverse reaction. Table 4 lists the most common (>1%) adverse reactions that resulted in discontinuation or dose reduction and that occurred more frequently in levetiracetam-treated patients than in placebo-treated patients.
Table 4: Adverse Reactions that Resulted in Discontinuation or Dose Reduction in Pooled Placebo-Controlled Studies in Adults Experiencing Partial-Onset Seizures Adverse Reaction
Levetiracetam
(N=769)
%
Placebo
(N=439)
%
Somnolence
4
2
Dizziness
1
0
Pediatric Patients 4 Years to <16 Years
The adverse reaction data presented below was obtained from a pooled analysis of two controlled pediatric clinical studies using an oral formulation in pediatric patients 4 to 16 years of age with partial-onset seizures. The most common adverse reactions in pediatric patients receiving levetiracetam in combination with other AEDs, for events with rates greater than placebo, were fatigue, aggression, nasal congestion, decreased appetite, and irritability.
Table 5 lists adverse reactions from the pooled pediatric controlled studies (4 to 16 years of age) that occurred in at least 2% of pediatric levetiracetam-treated patients and were numerically more common than in pediatric patients treated with placebo. In these studies, either levetiracetam or placebo was added to concurrent AED therapy.
Table 5: Adverse Reactions* in Pooled Placebo-Controlled, Adjunctive Studies in Pediatric Patients Ages 4 to 16 Years Experiencing Partial-Onset Seizures Levetiracetam
(N=165)
%
Placebo
(N=131)
%
- Headache
19
15
- Nasopharyngitis
15
12
- Vomiting
15
12
- Somnolence
13
9
- Fatigue
11
5
- Aggression
10
5
- Upper Abdominal Pain
9
8
- Cough
9
5
- Nasal Congestion
9
2
- Decreased Appetite
8
2
- Abnormal Behavior
7
4
- Dizziness
7
5
- Irritability
7
1
- Pharyngolaryngeal Pain
7
4
- Diarrhea
6
2
- Lethargy
6
5
- Insomnia
5
3
- Agitation
4
1
- Anorexia
4
3
- Head Injury
4
0
- Constipation
3
1
- Contusion
3
1
- Depression
3
1
- Fall
3
2
- Influenza
3
1
- Mood Altered
3
1
- Affect Lability
2
1
- Anxiety
2
1
- Arthralgia
2
0
- Confusional State
2
0
- Conjunctivitis
2
0
- Ear Pain
2
1
- Gastroenteritis
2
0
- Joint Sprain
2
1
- Mood Swings
2
1
- Neck Pain
2
1
- Rhinitis
2
0
- Sedation
2
1
* Adverse reactions occurred in at least 2% of pediatric levetiracetam-treated patients and occurred more frequently than placebo-treated patients
In the controlled pooled pediatric clinical studies in patients 4-16 years of age, 7% of patients receiving levetiracetam and 9% receiving placebo discontinued as a result of an adverse reaction.
Pediatric Patients 1 Month to < 4 Years
In the 7-day controlled pediatric clinical study using an oral formulation of levetiracetam in children 1 month to less than 4 years of age with partial-onset seizures, the most common adverse reactions in patients receiving levetiracetam in combination with other AEDs, for events with rates greater than placebo, were somnolence and irritability. Because of the shorter exposure period, incidences of adverse reactions are expected to be lower than in other pediatric studies in older patients. Therefore, other controlled pediatric data, presented above, should also be considered to apply to this age group.
Table 6 lists adverse reactions that occurred in at least 5% of pediatric epilepsy patients (ages 1 month to < 4 years) treated with levetiracetam in the placebo-controlled study and were numerically more common than in patients treated with placebo. In this study, either levetiracetam or placebo was added to concurrent AED therapy.
Table 6: Adverse Reactions* in a Placebo-Controlled, Adjunctive Study in Pediatric Patients Ages 1 Month to < 4 Years Experiencing Partial-Onset Seizures Levetiracetam
(N=60)
%
Placebo
(N=56)
%
- Somnolence
13
2
- Irritability
12
0
* Adverse reactions occurred in at least 5% of levetiracetam-treated patients and occurred more frequently than placebo-treated patients
In the 7-day controlled pediatric clinical study in patients 1 month to < 4 years of age, 3% of patients receiving levetiracetam and 2% receiving placebo either discontinued or had a dose reduction as a result of an adverse reaction. There was no adverse reaction that resulted in discontinuation for more than one patient.
Myoclonic Seizures
Although the pattern of adverse reactions in this study seems somewhat different from that seen in patients with partial-onset seizures, this is likely due to the much smaller number of patients in this study compared to partial seizure studies. The adverse reaction pattern for patients with JME is expected to be essentially the same as for patients with partial seizures.
In the controlled clinical study using levetiracetam tablets in patients with myoclonic seizures [see Clinical Studies (14.2)], the most common adverse reactions in patients receiving levetiracetam in combination with other AEDs, for events with rates greater than placebo, were somnolence, neck pain, and pharyngitis.
Table 7 lists adverse reactions that occurred in at least 5% of juvenile myoclonic epilepsy patients experiencing myoclonic seizures treated with levetiracetam tablets and were numerically more common than in patients treated with placebo. In this study, either levetiracetam or placebo was added to concurrent AED therapy.
Table 7: Adverse Reactions* in a Placebo-Controlled, Adjunctive Study in Patients 12 Years of Age and Older with Myoclonic Seizures Levetiracetam
(N=60)
%
Placebo
(N=60)
%
- Somnolence
12
2
- Neck pain
8
2
- Pharyngitis
7
0
- Depression
5
2
- Influenza
5
2
- Vertigo
5
3
* Adverse reactions occurred in at least 5% of levetiracetam-treated patients and occurred more frequently than placebo-treated patients
In the placebo-controlled study using levetiracetam tablets in patients with JME, 8% of patients receiving levetiracetam and 2% receiving placebo either discontinued or had a dose reduction as a result of an adverse reaction. The adverse reactions that led to discontinuation or dose reduction and that occurred more frequently in levetiracetam-treated patients than in placebo-treated patients are presented in Table 8.
Table 8: Adverse Reactions that Resulted in Discontinuation or Dose Reduction in Patients with Juvenile Myoclonic Epilepsy Adverse Reaction
Levetiracetam
(N=60)
%
Placebo
(N=60)
%
- Anxiety
3
2
- Depressed mood
2
0
- Depression
2
0
- Diplopia
2
0
- Hypersomnia
2
0
- Insomnia
2
0
- Irritability
2
0
- Nervousness
2
0
- Somnolence
2
0
Primary Generalized Tonic-Clonic Seizures
Although the pattern of adverse reactions in this study seems somewhat different from that seen in patients with partial seizures, this is likely due to the much smaller number of patients in this study compared to partial seizure studies. The adverse reaction pattern for patients with primary generalized tonic-clonic (PGTC) seizures is expected to be essentially the same as for patients with partial seizures.
In the controlled clinical study that included patients 4 years of age and older with PGTC seizures, the most common adverse reaction in patients receiving levetiracetam oral formulation in combination with other AEDs, for events with rates greater than placebo was nasopharyngitis.
Table 9 lists adverse reactions that occurred in at least 5% of idiopathic generalized epilepsy patients experiencing PGTC seizures treated with levetiracetam and were numerically more common than in patients treated with placebo. In this study, either levetiracetam or placebo was added to concurrent AED therapy.
Table 9: Adverse Reactions* in a Placebo-Controlled, Adjunctive Study in Patients 4 Years of Age and Older with PGTC Seizures Levetiracetam
(N=79)
%
Placebo
(N=84)
%
- Nasopharyngitis
14
5
- Fatigue
10
8
- Diarrhea
8
7
- Irritability
6
2
- Mood swings
5
1
* Adverse reactions occurred in at least 5% of levetiracetam-treated patients and occurred more frequently than placebo-treated patients
In the placebo-controlled study, 5% of patients receiving levetiracetam and 8% receiving placebo either discontinued or had a dose reduction during the treatment period as a result of an adverse reaction.
This study was too small to adequately characterize the adverse reactions that could be expected to result in discontinuation of treatment in this population. It is expected that the adverse reactions that would lead to discontinuation in this population would be similar to those resulting in discontinuation in other epilepsy trials (see tables 4 and 8).
In addition, the following adverse reactions were seen in other controlled adult studies of levetiracetam: balance disorder, disturbance in attention, eczema, memory impairment, myalgia, and blurred vision.
Comparison of Gender, Age and Race
The overall adverse reaction profile of levetiracetam was similar between females and males. There are insufficient data to support a statement regarding the distribution of adverse reactions by age and race.
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of levetiracetam. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been reported in patients receiving levetiracetam worldwide. The listing is alphabetized: abnormal liver function test, acute kidney injury, anaphylaxis, angioedema, agranulocytosis, choreoathetosis, drug reaction with eosinophilia and systemic symptoms (DRESS), dyskinesia, erythema multiforme, hepatic failure, hepatitis, hyponatremia, muscular weakness, obsessive-compulsive disorders (OCD), pancreatitis, pancytopenia (with bone marrow suppression identified in some of these cases), panic attack, thrombocytopenia, weight loss, and worsening of seizures including in patients with SCN8A mutations. Alopecia has been reported with levetiracetam use; recovery was observed in majority of cases where levetiracetam was discontinued.
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), including levetiracetam ...
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), including levetiracetam, during pregnancy. Encourage women who are taking levetiracetam during pregnancy to enroll in the North American Antiepileptic Drug (NAAED) pregnancy registry by calling 1-888-233-2334 or visiting http://www.aedpregnancyregistry.org/.
Risk Summary
Prolonged experience with levetiracetam in pregnant women has not identified a drug-associated risk of major birth defects or miscarriage, based on published literature, which includes data from pregnancy registries, and reflects experience over two decades [see Human Data]. In animal studies, levetiracetam produced developmental toxicity (increased embryofetal and offspring mortality, increased incidences of fetal structural abnormalities, decreased embryofetal and offspring growth, neurobehavioral alterations in offspring) at doses similar to human therapeutic doses [see Animal Data].
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Clinical Considerations
Levetiracetam blood levels may decrease during pregnancy [see Warnings and Precautions (5.10)].
Physiological changes during pregnancy may affect levetiracetam concentration. Decrease in levetiracetam plasma concentrations has been observed during pregnancy. This decrease is more pronounced during the third trimester. Dose adjustments may be necessary to maintain clinical response.
Data
Human Data
While available studies cannot definitively establish the absence of risk, data from the published literature and pregnancy registries have not established an association with levetiracetam use during pregnancy and major birth defects or miscarriage.
Animal Data
When levetiracetam (0, 400, 1,200, or 3,600 mg/kg/day) was administered orally to pregnant rats during the period of organogenesis, reduced fetal weights and increased incidence of fetal skeletal variations were observed at the highest dose tested. There was no evidence of maternal toxicity. The no-effect dose for adverse effects on embryofetal developmental in rats (1,200 mg/kg/day) is approximately 4 times the maximum recommended human dose (MRHD) of 3,000 mg on a body surface area (mg/m2) basis.
Oral administration of levetiracetam (0, 200, 600, or 1,800 mg/kg/day) to pregnant rabbits during the period of organogenesis resulted in increased embryofetal mortality and incidence of fetal skeletal variations at the mid and high dose and decreased fetal weights and increased incidence of fetal malformations at the high dose, which was associated with maternal toxicity. The no-effect dose for adverse effects on embryofetal development in rabbits (200 mg/kg/day) is approximately equivalent to the MRHD on a mg/m2 basis.
Oral administration of levetiracetam (0, 70, 350, or 1,800 mg/kg/day) to female rats throughout pregnancy and lactation led to an increased incidence of fetal skeletal variations, reduced fetal body weight, and decreased growth in offspring at the mid and high doses and increased pup mortality and neurobehavioral alterations in offspring at the highest dose tested. There was no evidence of maternal toxicity. The no-effect dose for adverse effects on pre-and postnatal development in rats (70 mg/kg/day) is less than the MRHD on a mg/m2 basis.
Oral administration of levetiracetam to rats during the latter part of gestation and throughout lactation produced no adverse developmental or maternal effects at doses of up to 1,800 mg/kg/day (6 times the MRHD on a mg/m2 basis).
8.2 Lactation
Risk Summary
Levetiracetam is excreted in human milk. There are no data on the effects of levetiracetam on the breastfed infant, or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for levetiracetam and any potential adverse effects on the breastfed infant from levetiracetam or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of levetiracetam for the treatment of partial-onset seizures in patients 1 month to 16 years of age have been established [see Clinical Pharmacology (12.3) and Clinical Studies (14.1)]. The dosing recommendation in these pediatric patients varies according to age group and is weight-based [see Dosage and Administration (2.6)].
The safety and effectiveness of levetiracetam as adjunctive therapy for the treatment of myoclonic seizures in adolescents 12 years of age and older with juvenile myoclonic epilepsy have been established [see Clinical Studies (14.2)].
The safety and effectiveness of levetiracetam as adjunctive therapy for the treatment of primary generalized tonic-clonic seizures in pediatric patients 6 years of age and older with idiopathic generalized epilepsy have been established [see Clinical Studies (14.3)].
Safety and effectiveness for the treatment of partial-onset seizures in pediatric patients below the age of 1 month; adjunctive therapy for the treatment of myoclonic seizures in pediatric patients below the age of 12 years; and adjunctive therapy for the treatment of primary generalized tonic-clonic seizures in pediatric patients below the age of 6 years have not been established.
A 3-month, randomized, double-blind, placebo-controlled study was performed to assess the neurocognitive and behavioral effects of levetiracetam as adjunctive therapy in 98 (levetiracetam N=64, placebo N=34) pediatric patients, ages 4 years to 16 years, with partial seizures that were inadequately controlled. The target dose was 60 mg/kg/day. Neurocognitive effects were measured by the Leiter-R Attention and Memory (AM) Battery, which measures various aspects of a child's memory and attention. Although no substantive differences were observed between the placebo and drug treated groups in the median change from baseline in this battery, the study was not adequate to assess formal statistical non-inferiority of the drug and placebo. The Achenbach Child Behavior Checklist (CBCL/6-18), a standardized validated tool used to assess a child’s competencies and behavioral/emotional problems, was also assessed in this study. An analysis of the CBCL/6-18 indicated, on average, a worsening in levetiracetam-treated patients in aggressive behavior, one of the eight syndrome scores [see Warnings and Precautions (5.1)].
Juvenile Animal Toxicity Data
Studies of levetiracetam in juvenile rats (dosed on postnatal days 4 through 52) and dogs (dosed from postnatal weeks 3 through 7) at doses of up to 1,800 mg/kg/day (approximately 7 and 24 times, respectively, the maximum recommended pediatric dose of 60 mg/kg/day on a mg/m2 basis) did not demonstrate adverse effects on postnatal development.
8.5 Geriatric Use
There were 347 subjects in clinical studies of levetiracetam that were 65 years old and over. No overall differences in safety were observed between these subjects and younger subjects. There were insufficient numbers of elderly subjects in controlled trials of epilepsy to adequately assess the effectiveness of levetiracetam in these patients.
Levetiracetam is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Clinical Pharmacology (12.3)].
Close8.6 Renal Impairment
Clearance of levetiracetam is decreased in patients with renal impairment and is correlated with creatinine clearance [see Clinical Pharmacology (12.3)]. Dosage adjustment is recommended for patients with impaired renal function and supplemental doses should be given to patients after dialysis [see Dosage and Administration (2.7)].
-
10 OVERDOSAGE 10.1 Signs, Symptoms and Laboratory Findings of Acute Overdosage in Humans - The highest known dose of oral levetiracetam received in the clinical development program was 6000 mg/day. Other than ...
10.1 Signs, Symptoms and Laboratory Findings of Acute Overdosage in Humans
The highest known dose of oral levetiracetam received in the clinical development program was 6000 mg/day. Other than drowsiness, there were no adverse reactions in the few known cases of overdose in clinical trials. Cases of somnolence, agitation, aggression, depressed level of consciousness, respiratory depression, and coma were observed with levetiracetam overdoses in postmarketing use.
10.2 Management of Overdose
There is no specific antidote for overdose with levetiracetam. If indicated, elimination of unabsorbed drug should be attempted by emesis or gastric lavage; usual precautions should be observed to maintain airway. General supportive care of the patient is indicated including monitoring of vital signs and observation of the patient’s clinical status. A Certified Poison Control Center should be contacted for up to date information on the management of overdose with levetiracetam.
Close10.3 Hemodialysis
Standard hemodialysis procedures result in significant clearance of levetiracetam (approximately 50% in 4 hours) and should be considered in cases of overdose. Although hemodialysis has not been performed in the few known cases of overdose, it may be indicated by the patient's clinical state or in patients with significant renal impairment.
-
11 DESCRIPTION Levetiracetam injection is an antiepileptic drug available as a clear, colorless, sterile solution (100 mg/mL) for intravenous administration. The chemical name of levetiracetam, a single ...
Levetiracetam injection is an antiepileptic drug available as a clear, colorless, sterile solution (100 mg/mL) for intravenous administration.
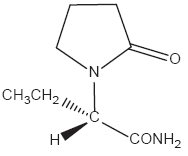
The chemical name of levetiracetam, a single enantiomer, is (-)-(S)-α-ethyl-2-oxo-1-pyrrolidine acetamide, its molecular formula is C8H14N2O2 and its molecular weight is 170.21. Levetiracetam is chemically unrelated to existing antiepileptic drugs (AEDs). It has the following structural formula:
Levetiracetam is a white to off-white crystalline powder with a faint odor and a bitter taste. It is very soluble in water (104.0 g/100 mL). It is freely soluble in chloroform (65.3 g/100 mL) and in methanol (53.6 g/100 mL), soluble in ethanol (16.5 g/100 mL), sparingly soluble in acetonitrile (5.7 g/100 mL) and practically insoluble in n-hexane. (Solubility limits are expressed as g/100 mL solvent.)
Levetiracetam injection, USP contains 100 mg of levetiracetam, USP per mL. It is supplied in single-dose 5 mL vials containing 500 mg levetiracetam, water for injection, 45 mg sodium chloride, and buffered at approximately pH 5.5 with glacial acetic acid and 8.2 mg sodium acetate trihydrate. Levetiracetam injection must be diluted prior to intravenous infusion [see Dosage and Administration (2.6)].
Close -
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - The precise mechanism(s) by which levetiracetam exerts its antiepileptic effect is unknown. A saturable and stereoselective neuronal binding site in rat brain tissue ...
12.1 Mechanism of Action
The precise mechanism(s) by which levetiracetam exerts its antiepileptic effect is unknown.
A saturable and stereoselective neuronal binding site in rat brain tissue has been described for levetiracetam. Experimental data indicate that this binding site is the synaptic vesicle protein SV2A, thought to be involved in the regulation of vesicle exocytosis. Although the molecular significance of levetiracetam binding to synaptic vesicle protein SV2A is not understood, levetiracetam and related analogs showed a rank order of affinity for SV2A which correlated with the potency of their antiseizure activity in audiogenic seizure-prone mice. These findings suggest that the interaction of levetiracetam with the SV2A protein may contribute to the antiepileptic mechanism of action of the drug.
12.2 Pharmacodynamics
Effects on QTc Interval
The effect of levetiracetam on QTc prolongation was evaluated in a randomized, double-blind, positive-controlled (moxifloxacin 400 mg) and placebo-controlled crossover study of levetiracetam (1,000 mg or 5,000 mg) in 52 healthy subjects. The upper bound of the 90% confidence interval for the largest placebo-adjusted, baseline-corrected QTc was below 10 milliseconds. Therefore, there was no evidence of significant QTc prolongation in this study.
Close12.3 Pharmacokinetics
Equivalent doses of intravenous (IV) levetiracetam and oral levetiracetam result in equivalent Cmax, Cmin, and total systemic exposure to levetiracetam when the intravenous levetiracetam is administered as a 15-minute infusion.
Overview
Levetiracetam is rapidly and almost completely absorbed after oral administration. Levetiracetam injection and tablets are bioequivalent. The pharmacokinetics of levetiracetam are linear and time-invariant, with low intra- and inter-subject variability. Levetiracetam is not significantly protein-bound (<10% bound) and its volume of distribution is close to the volume of intracellular and extracellular water. Sixty-six percent (66%) of the dose is renally excreted unchanged. The major metabolic pathway of levetiracetam (24% of dose) is an enzymatic hydrolysis of the acetamide group. It is not liver cytochrome P450 dependent. The metabolites have no known pharmacological activity and are renally excreted. Plasma half-life of levetiracetam across studies is approximately 6-8 hours. It is increased in the elderly (primarily due to impaired renal clearance) and in subjects with renal impairment.
The pharmacokinetics of levetiracetam are similar when used as monotherapy or as adjunctive therapy for the treatment of partial-onset seizures.
Distribution
The equivalence of levetiracetam injection and the oral formulation was demonstrated in a bioavailability study of 17 healthy volunteers. In this study, levetiracetam 1,500 mg was diluted in 100 mL 0.9% sterile saline solution and was infused over 15 minutes. The selected infusion rate provided plasma concentrations of levetiracetam at the end of the infusion period similar to those achieved at Tmax after an equivalent oral dose. It is demonstrated that levetiracetam 1,500 mg intravenous infusion is equivalent to levetiracetam 3 x 500 mg oral tablets. The time independent pharmacokinetic profile of levetiracetam was demonstrated following 1,500 mg intravenous infusion for 4 days with BID dosing. The AUC(0-12) at steady-state was equivalent to AUCinf following an equivalent single dose.
Levetiracetam and its major metabolite are less than 10% bound to plasma proteins; clinically significant interactions with other drugs through competition for protein binding sites are therefore unlikely.
Metabolism
Levetiracetam is not extensively metabolized in humans. The major metabolic pathway is the enzymatic hydrolysis of the acetamide group, which produces the carboxylic acid metabolite, ucb L057 (24% of dose) and is not dependent on any liver cytochrome P450 isoenzymes. The major metabolite is inactive in animal seizure models. Two minor metabolites were identified as the product of hydroxylation of the 2-oxo-pyrrolidine ring (2% of dose) and opening of the 2-oxo-pyrrolidine ring in position 5 (1% of dose). There is no enantiomeric interconversion of levetiracetam or its major metabolite.
Elimination
Levetiracetam plasma half-life in adults is 7 ± 1 hour and is unaffected by either dose, route of administration or repeated administration. Levetiracetam is eliminated from the systemic circulation by renal excretion as unchanged drug which represents 66% of administered dose. The total body clearance is 0.96 mL/min/kg and the renal clearance is 0.6 mL/min/kg. The mechanism of excretion is glomerular filtration with subsequent partial tubular reabsorption. The metabolite ucb L057 is excreted by glomerular filtration and active tubular secretion with a renal clearance of 4 mL/min/kg. Levetiracetam elimination is correlated to creatinine clearance. Levetiracetam clearance is reduced in patients with renal impairment [see Dosage and Administration (2.6) and Use in Specific Populations (8.6)].
Specific Populations
Elderly
Pharmacokinetics of levetiracetam were evaluated in 16 elderly subjects (age 61-88 years) with creatinine clearance ranging from 30 to 74 mL/min. Following oral administration of twice-daily dosing for 10 days, total body clearance decreased by 38% and the half-life was 2.5 hours longer in the elderly compared to healthy adults. This is most likely due to the decrease in renal function in these subjects.
Pediatric Patients
- •
- Intravenous Formulation
- A population pharmacokinetic analysis for the intravenous formulation was conducted in 49 pediatric patients (1 month to < 16 years of age) weighing 3-79 kg. Patients received levetiracetam as a 15-minute intravenous infusion at doses between 14 mg/kg/day and 60 mg/kg/day twice daily. Plasma concentrations and model derived steady-state exposure AUC (0-12) were within the range of the exposure observed in pediatric patients receiving equivalent doses of the oral solution.
- •
- Oral Formulations
- Pharmacokinetics of levetiracetam were evaluated in 24 pediatric patients (age 6-12 years) after single oral dose (20 mg/kg) of the immediate release formulation of levetiracetam. The body weight adjusted apparent clearance of levetiracetam was approximately 40% higher than in adults.
- A repeat dose pharmacokinetic study was conducted in pediatric patients (age 4-12 years) at doses of 20 mg/kg/day, 40 mg/kg/day, and 60 mg/kg/day of the immediate release formulation of levetiracetam. The evaluation of the pharmacokinetic profile of levetiracetam and its metabolite (ucb L057) in 14 pediatric patients demonstrated rapid absorption of levetiracetam at all doses, with a Tmax of about 1 hour and a t1/2 of 5 hours across all dosing levels. The pharmacokinetics of levetiracetam in pediatric patients was linear between 20 to 60 mg/kg/day. The potential interaction of levetiracetam with other AEDs was also evaluated in these patients. Levetiracetam had no significant effect on the plasma concentrations of carbamazepine, valproic acid, topiramate or lamotrigine. However, there was about a 22% increase of apparent clearance of levetiracetam when it was co-administered with an enzyme-inducing AED (e.g., carbamazepine).
- Following single dose administration (20 mg/kg) of a 10% oral solution to pediatric patients with epilepsy (1 month to < 4 years), levetiracetam was rapidly absorbed and peak plasma concentrations were observed approximately 1 hour after dosing. Levetiracetam half-life in pediatric patients 1 month to < 4 years with epilepsy was shorter (5.3 h) than in adults (7.2 h), and apparent clearance (1.5 mL/min/kg) was faster than in adults (0.96 mL/min/kg).
- Population pharmacokinetic analysis showed that body weight was significantly correlated to the clearance of levetiracetam in pediatric patients; clearance increased with an increase in body weight.
Pregnancy
Levetiracetam levels may decrease during pregnancy [see Warnings and Precautions (5.10) and Use in Specific Populations (8.1)].
Gender
Levetiracetam Cmax and AUC were 20% higher in women (N=11) compared to men (N=12). However, clearances adjusted for body weight were comparable.
Race
Formal pharmacokinetic studies of the effects of race have not been conducted. Cross-study comparisons involving Caucasians (N=12) and Asians (N=12), however, show that pharmacokinetics of levetiracetam were comparable between the two races. Because levetiracetam is primarily renally excreted and there are no important racial differences in creatinine clearance, pharmacokinetic differences due to race are not expected.
Renal Impairment
The disposition of levetiracetam was studied in adult subjects with varying degrees of renal function. Total body clearance of levetiracetam is reduced in patients with impaired renal function by 40% in the mild group (CLcr = 50-80 mL/min), 50% in the moderate group (CLcr = 30-50 mL/min) and 60% in the severe renal impairment group (CLcr <30 mL/min). Clearance of levetiracetam is correlated with creatinine clearance.
In anuric (end stage renal disease) patients, the total body clearance decreased 70% compared to normal subjects (CLcr >80mL/min). Approximately 50% of the pool of levetiracetam in the body is removed during a standard 4 hour hemodialysis procedure [see Dosage and Administration (2.7)].
Hepatic Impairment
In subjects with mild (Child-Pugh A) to moderate (Child-Pugh B) hepatic impairment, the pharmacokinetics of levetiracetam were unchanged. In patients with severe hepatic impairment (Child-Pugh C), total body clearance was 50% that of normal subjects, but decreased renal clearance accounted for most of the decrease. No dose adjustment is needed for patients with hepatic impairment.
Drug Interactions
In vitro data on metabolic interactions indicate that levetiracetam is unlikely to produce, or be subject to, pharmacokinetic interactions. Levetiracetam and its major metabolite, at concentrations well above Cmax levels achieved within the therapeutic dose range, are neither inhibitors of, nor high affinity substrates for, human liver cytochrome P450 isoforms, epoxide hydrolase or UDP-glucuronidation enzymes. In addition, levetiracetam does not affect the in vitro glucuronidation of valproic acid.
Potential pharmacokinetic interactions of or with levetiracetam were assessed in clinical pharmacokinetic studies (phenytoin, valproate, warfarin, digoxin, oral contraceptive, probenecid) and through pharmacokinetic screening in the placebo-controlled clinical studies in epilepsy patients.
Phenytoin
Levetiracetam (3,000 mg daily) had no effect on the pharmacokinetic disposition of phenytoin in patients with refractory epilepsy. Pharmacokinetics of levetiracetam were also not affected by phenytoin.
Valproate
Levetiracetam (1,500 mg twice daily) did not alter the pharmacokinetics of valproate in healthy volunteers. Valproate 500 mg twice daily did not modify the rate or extent of levetiracetam absorption or its plasma clearance or urinary excretion. There also was no effect on exposure to and the excretion of the primary metabolite, ucb L057.
Other Antiepileptic Drugs
Potential drug interactions between levetiracetam and other AEDs (carbamazepine, gabapentin, lamotrigine, phenobarbital, phenytoin, primidone and valproate) were also assessed by evaluating the serum concentrations of levetiracetam and these AEDs during placebo-controlled clinical studies. These data indicate that levetiracetam does not influence the plasma concentration of other AEDs and that these AEDs do not influence the pharmacokinetics of levetiracetam.
Effect of AEDs in Pediatric Patients
There was about a 22% increase of apparent total body clearance of levetiracetam when it was co-administered with enzyme-inducing AEDs. Dose adjustment is not recommended. Levetiracetam had no effect on plasma concentrations of carbamazepine, valproate, topiramate, or lamotrigine.
Oral Contraceptives
Levetiracetam (500 mg twice daily) did not influence the pharmacokinetics of an oral contraceptive containing 0.03 mg ethinyl estradiol and 0.15 mg levonorgestrel, or of the luteinizing hormone and progesterone levels, indicating that impairment of contraceptive efficacy is unlikely. Coadministration of this oral contraceptive did not influence the pharmacokinetics of levetiracetam.
Digoxin
Levetiracetam (1,000 mg twice daily) did not influence the pharmacokinetics and pharmacodynamics (ECG) of digoxin given as a 0.25 mg dose every day. Coadministration of digoxin did not influence the pharmacokinetics of levetiracetam.
Warfarin
Levetiracetam (1,000 mg twice daily) did not influence the pharmacokinetics of R and S warfarin. Prothrombin time was not affected by levetiracetam. Coadministration of warfarin did not affect the pharmacokinetics of levetiracetam.
Probenecid
Probenecid, a renal tubular secretion blocking agent, administered at a dose of 500 mg four times a day, did not change the pharmacokinetics of levetiracetam 1,000 mg twice daily. Cssmax of the metabolite, ucb L057, was approximately doubled in the presence of probenecid while the fraction of drug excreted unchanged in the urine remained the same. Renal clearance of ucb L057 in the presence of probenecid decreased 60%, probably related to competitive inhibition of tubular secretion of ucb L057. The effect of levetiracetam on probenecid was not studied.
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Rats were dosed with levetiracetam in the diet for 104 weeks at doses of 50, 300, and 1,800 mg/kg/day. Plasma ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Rats were dosed with levetiracetam in the diet for 104 weeks at doses of 50, 300, and 1,800 mg/kg/day. Plasma exposure (AUC) at the highest dose was approximately 6 times that in humans at the maximum recommended human dose (MRHD) of 3,000 mg. There was no evidence of carcinogenicity. In mice, oral administration of levetiracetam for 80 weeks (doses up to 960 mg/kg/day) or 2 years (doses up to 4,000 mg/kg/day, lowered to 3,000 mg/kg/day after 45 weeks due to intolerability) was not associated with an increase in tumors. The highest dose tested in mice for 2 years (3,000 mg/kg/day) is approximately 5 times the MRHD on a body surface area (mg/m2) basis.
Mutagenesis
Levetiracetam was negative in in vitro (Ames, chromosomal aberration in mammalian cells) and in vivo (mouse micronucleus) assays. The major human metabolite of levetiracetam (ucb L057) was negative in in vitro (Ames, mouse lymphoma) assays.
Impairment of Fertility
No adverse effects on male or female fertility or reproductive performance were observed in rats at oral doses up to 1,800 mg/kg/day, which were associated with plasma exposures (AUC) up to approximately 6 times that in humans at the MRHD.
-
14 CLINICAL STUDIES All clinical studies supporting the efficacy of levetiracetam utilized oral formulations. The finding of efficacy of levetiracetam injection is based on the results of studies using an oral ...
All clinical studies supporting the efficacy of levetiracetam utilized oral formulations. The finding of efficacy of levetiracetam injection is based on the results of studies using an oral formulation of levetiracetam, and on the demonstration of comparable bioavailability of the oral and parenteral formulations [see Clinical Pharmacology (12.3)].
14.1 Partial-Onset Seizures
Effectiveness in Partial-Onset Seizures in Adults
The effectiveness of levetiracetam for the treatment of partial-onset seizures in adults was established in three multicenter, randomized, double-blind, placebo-controlled clinical studies in patients who had refractory partial-onset seizures with or without secondary generalization. The tablet formulation was used in all these studies. In these studies, 904 patients were randomized to placebo, 1,000 mg, 2,000 mg, or 3,000 mg/day. Patients enrolled in Study 1 or Study 2 had refractory partial-onset seizures for at least two years and had taken two or more classical AEDs. Patients enrolled in Study 3 had refractory partial-onset seizures for at least 1 year and had taken one classical AED. At the time of the study, patients were taking a stable dose regimen of at least one and could take a maximum of two AEDs. During the baseline period, patients had to have experienced at least two partial-onset seizures during each 4-week period.
Study 1
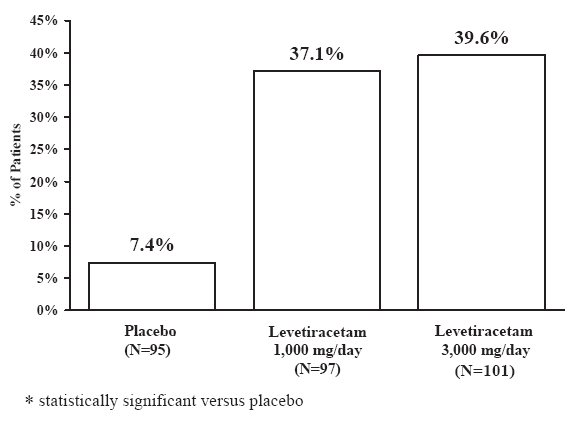
Study 1 was a double-blind, placebo-controlled, parallel-group study conducted at 41 sites in the United States comparing levetiracetam 1,000 mg/day (N=97), levetiracetam 3,000 mg/day (N=101), and placebo (N=95) given in equally divided doses twice daily. After a prospective baseline period of 12 weeks, patients were randomized to one of the three treatment groups described above. The 18-week treatment period consisted of a 6-week titration period, followed by a 12-week fixed dose evaluation period, during which concomitant AED regimens were held constant. The primary measure of effectiveness was a between group comparison of the percent reduction in weekly partial seizure frequency relative to placebo over the entire randomized treatment period (titration + evaluation period). Secondary outcome variables included the responder rate (incidence of patients with ≥50% reduction from baseline in partial-onset seizure frequency). The results of the analysis of Study 1 are displayed in Table 10.
Table 10: Reduction in Mean Over Placebo in Weekly Frequency of Partial-Onset Seizures in Study 1
Placebo
(N=95)
Levetiracetam
1,000 mg/day
(N=97)
Levetiracetam
3,000 mg/day
(N=101)
Percent reduction in partial seizure frequency over placebo
–
26.1%*
30.1%*
* statistically significant versus placebo
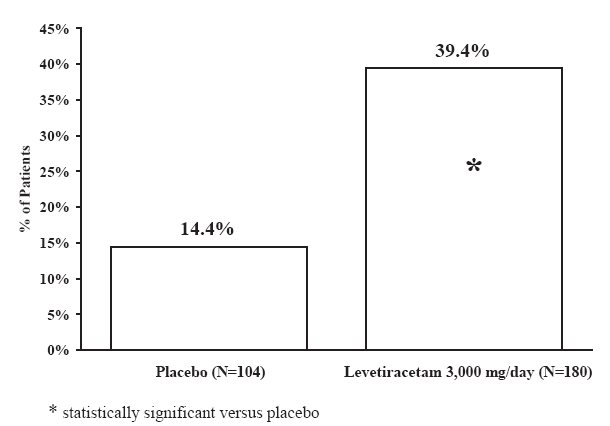
The percentage of patients (y-axis) who achieved ≥50% reduction in weekly seizure rates from baseline in partial-onset seizure frequency over the entire randomized treatment period (titration + evaluation period) within the three treatment groups (x-axis) is presented in Figure 1.
Figure 1: Responder Rate (≥50% Reduction from Baseline) in Study 1
Study 2
Study 2 was a double-blind, placebo-controlled, crossover study conducted at 62 centers in Europe comparing levetiracetam 1,000 mg/day (N=106), levetiracetam 2,000 mg/day (N=105), and placebo (N=111) given in equally divided doses twice daily.
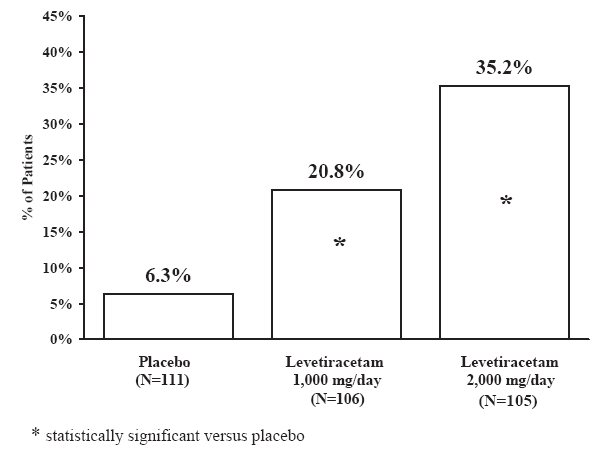
The first period of the study (Period A) was designed to be analyzed as a parallel-group study. After a prospective baseline period of up to 12 weeks, patients were randomized to one of the three treatment groups described above. The 16-week treatment period consisted of the 4-week titration period followed by a 12-week fixed dose evaluation period, during which concomitant AED regimens were held constant. The primary measure of effectiveness was a between group comparison of the percent reduction in weekly partial seizure frequency relative to placebo over the entire randomized treatment period (titration + evaluation period). Secondary outcome variables included the responder rate (incidence of patients with ≥50% reduction from baseline in partial-onset seizure frequency). The results of the analysis of Period A are displayed in Table 11.
Table 11: Reduction in Mean Over Placebo in Weekly Frequency of Partial-Onset Seizures in Study 2: Period A
Placebo
(N=111)
Levetiracetam
1,000 mg/day
(N=106)
Levetiracetam
2,000 mg/day
(N=105)
Percent reduction in partial seizure frequency over placebo
–
17.1%*
21.4%*
* statistically significant versus placebo
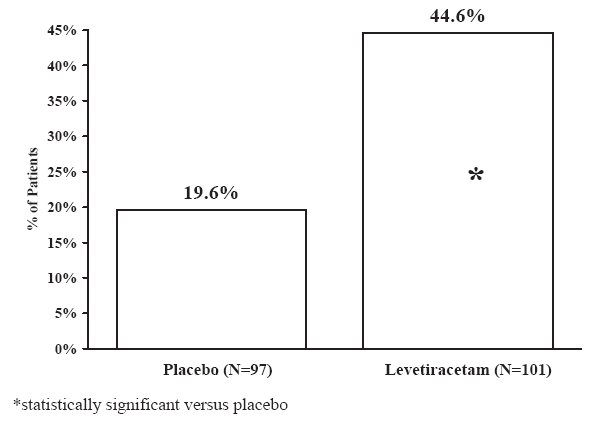
The percentage of patients (y-axis) who achieved ≥50% reduction in weekly seizure rates from baseline in partial-onset seizure frequency over the entire randomized treatment period (titration + evaluation period) within the three treatment groups (x-axis) is presented in Figure 2.
Figure 2: Responder Rate (≥50% Reduction from Baseline) in Study 2: Period A
The comparison of levetiracetam 2,000 mg/day to levetiracetam 1,000 mg/day for responder rate was statistically significant (P=0.02). Analysis of the trial as a cross-over yielded similar results.
Study 3
Study 3 was a double-blind, placebo-controlled, parallel-group study conducted at 47 centers in Europe comparing levetiracetam 3,000 mg/day (N=180) and placebo (N=104) in patients with refractory partial-onset seizures, with or without secondary generalization, receiving only one concomitant AED. Study drug was given in two divided doses. After a prospective baseline period of 12 weeks, patients were randomized to one of two treatment groups described above. The 16-week treatment period consisted of a 4-week titration period, followed by a 12-week fixed dose evaluation period, during which concomitant AED doses were held constant. The primary measure of effectiveness was a between group comparison of the percent reduction in weekly seizure frequency relative to placebo over the entire randomized treatment period (titration + evaluation period). Secondary outcome variables included the responder rate (incidence of patients with ≥50% reduction from baseline in partial-onset seizure frequency). Table 12 displays the results of the analysis of Study 3.
Table 12: Reduction in Mean Over Placebo in Weekly Frequency of Partial-Onset Seizures in Study 3
Placebo
(N=104)
Levetiracetam
3,000 mg/day
(N=180)
Percent reduction in partial seizure frequency over placebo
–
23.0%*
* statistically significant versus placebo
The percentage of patients (y-axis) who achieved ≥50% reduction in weekly seizure rates from baseline in partial-onset seizure frequency over the entire randomized treatment period (titration + evaluation period) within the two treatment groups (x-axis) is presented in Figure 3.
Figure 3: Responder Rate (≥50% Reduction from Baseline) in Study 3
Effectiveness in Partial-Onset Seizures in Pediatric Patients 4 Years to 16 Years of Age
Study 4 was a multicenter, randomized double-blind, placebo-controlled study, in pediatric patients 4 to 16 years of age with partial seizures uncontrolled by standard antiepileptic drugs (AEDs). Study 4 was conducted at 60 sites in North America. The study consisted of an 8-week baseline period and 4-week titration period followed by a 10-week evaluation period. Eligible patients who still experienced, on a stable dose of 1-2 AEDs, at least 4 partial-onset seizures during the 4 weeks prior to screening, as well as at least 4 partial-onset seizures in each of the two 4-week baseline periods, were randomized to receive either levetiracetam or placebo. Dosing was initiated at a dose of 20 mg/kg/day in two divided doses. During the treatment period, levetiracetam doses were adjusted in 20 mg/kg/day increments, at 2-week intervals to the target dose of 60 mg/kg/day. The primary measure of efficacy was a between group comparison of the percent reduction in weekly partial seizure frequency relative to placebo over the entire 14-week randomized treatment period (titration + evaluation period). Secondary outcome variables included the responder rate (incidence of patients with ≥ 50% reduction from baseline in partial-onset seizure frequency per week). The enrolled population included 198 patients (levetiracetam N=101, placebo N=97) with refractory partial-onset seizures, whether or not secondarily generalized. Table 13 displays the results of Study 4.
Table 13: Reduction in Mean Over Placebo in Weekly Frequency of Partial-Onset Seizures in Study 4
Placebo
(N=97)
Levetiracetam
(N=101)
Percent reduction in partial seizure frequency over placebo
-
26.8%*
* statistically significant versus placebo
The percentage of patients (y-axis) who achieved ≥ 50% reduction in weekly seizure rates from baseline in partial-onset seizure frequency over the entire randomized treatment period (titration + evaluation period) within the two treatment groups (x-axis) is presented in Figure 4.
Figure 4: Responder Rate (≥ 50% Reduction from Baseline) in Study 4
Effectiveness in Partial-Onset Seizures in Pediatric Patients 1 Month to <4 Years of Age
Study 5 was a multicenter, randomized double-blind, placebo-controlled study, in pediatric patients 1 month to less than 4 years of age with partial seizures, uncontrolled by standard epileptic drugs (AEDs). Study 5 was conducted at 62 sites in North America, South America, and Europe. Study 5 consisted of a 5-day evaluation period, which included a 1-day titration period followed by a 4-day maintenance period. Eligible patients who experienced, on a stable dose of 1-2 AEDs, at least 2 partial-onset seizures during the 48-hour baseline video EEG were randomized to receive either levetiracetam or placebo. Randomization was stratified by age range as follows: 1 month to less than 6 months of age (N=4 treated with levetiracetam), 6 months to less than 1 year of age (N=8 treated with levetiracetam), 1 year to less than 2 years of age (N=20 treated with levetiracetam), and 2 years to less than 4 years of age (N=28 treated with levetiracetam). Levetiracetam dosing was determined by age and weight as follows: children 1 month to less than 6 months old were randomized to a target dose of 40 mg/kg/day, and children 6 months to less than 4 years old were randomized to a target dose of 50 mg/kg/day. The primary measure of efficacy was the responder rate (percent of patients with ≥ 50% reduction from baseline in average daily partial-onset seizure frequency) assessed by a blinded central reader using a 48-hour video EEG performed during the last two days of the 4-day maintenance period. The enrolled population included 116 patients (levetiracetam N=60, placebo N=56) with refractory partial-onset seizures, whether or not secondarily generalized. A total of 109 patients were included in the efficacy analysis. A statistically significant difference between levetiracetam and placebo was observed in Study 5 (see Figure 5). The treatment effect associated with levetiracetam was consistent across age groups.
Figure 5: Responder Rate for All Patients Ages 1 Month to < 4 Years (≥ 50% Reduction from Baseline) in Study 5
14.2 Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy
The effectiveness of levetiracetam as adjunctive therapy in patients 12 years of age and older with juvenile myoclonic epilepsy (JME) experiencing myoclonic seizures was established in one multicenter, randomized, double-blind, placebo-controlled study (study 6), conducted at 37 sites in 14 countries. Eligible patients on a stable dose of 1 antiepileptic drug (AED) experiencing one or more myoclonic seizures per day for at least 8 days during the prospective 8-week baseline period were randomized to either levetiracetam or placebo (levetiracetam N=60, placebo N=60). Patients were titrated over 4 weeks to a target dose of 3,000 mg/day and treated at a stable dose of 3,000 mg/day over 12 weeks (evaluation period). Study drug was given in 2 divided doses. The primary measure of efficacy was the proportion of patients with at least 50% reduction in the number of days per week with one or more myoclonic seizures during the treatment period (titration + evaluation periods) as compared to baseline. Table 14 displays the results for the 113 patients with JME in this study.
Table 14: Responder Rate (≥50% Reduction from Baseline) in Myoclonic Seizure Days per Week in Study 6
Placebo
(N=59)
Levetiracetam
(N=54)
Percentage of responders
23.7%
60.4%*
* statistically significant versus placebo
Close14.3 Primary Generalized Tonic-Clonic Seizures
The effectiveness of levetiracetam as adjunctive therapy in patients 6 years of age and older with idiopathic generalized epilepsy experiencing primary generalized tonic-clonic (PGTC) seizures was established in one multicenter, randomized, double-blind, placebo-controlled study (study 7), conducted at 50 sites in 8 countries. Eligible patients on a stable dose of 1 or 2 antiepileptic drugs (AEDs) experiencing at least 3 PGTC seizures during the 8-week combined baseline period (at least one PGTC seizure during the 4 weeks prior to the prospective baseline period and at least one PGTC seizure during the 4-week prospective baseline period) were randomized to either levetiracetam or placebo. The 8-week combined baseline period is referred to as “baseline” in the remainder of this section. Patients were titrated over 4 weeks to a target dose of 3,000 mg/day for adults or a pediatric target dose of 60 mg/kg/day and treated at a stable dose of 3,000 mg/day (or 60 mg/kg/day for children) over 20 weeks (evaluation period). Study drug was given in 2 equally divided doses per day. The primary measure of efficacy was the percent reduction from baseline in weekly PGTC seizure frequency for levetiracetam and placebo treatment groups over the treatment period (titration + evaluation periods). The population included 164 patients (levetiracetam N=80, placebo N=84) with idiopathic generalized epilepsy (predominately juvenile myoclonic epilepsy, juvenile absence epilepsy, childhood absence epilepsy, or epilepsy with Grand Mal seizures on awakening) experiencing primary generalized tonic-clonic seizures. Each of these syndromes of idiopathic generalized epilepsy was well represented in this patient population.
There was a statistically significant decrease from baseline in PGTC frequency in the levetiracetam-treated patients compared to the placebo-treated patients in Study 7 (see Table 15).
Table 15: Median Percent Reduction from Baseline in PGTC Seizure Frequency per Week in Study 7
Placebo
(N=84)
Levetiracetam
(N=78)
Percentage reduction in PGTC seizure frequency
44.6%
77.6%*
* statistically significant versus placebo
The percentage of patients (y-axis) who achieved ≥50% reduction in weekly seizure rates from baseline in PGTC seizure frequency over the entire randomized treatment period (titration + evaluation period) within the two treatment groups (x-axis) is presented in Figure 6.
Figure 6: Responder Rate (≥50% Reduction from Baseline) in PGTC Seizure Frequency per Week in Study 7
-
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied - Levetiracetam injection, USP 500 mg/5 mL (100 mg/mL) is a clear, colorless, sterile solution. It is supplied in single-dose 5 mL vials (NDC 43547-454-41), available in ...
16.1 How Supplied
Levetiracetam injection, USP 500 mg/5 mL (100 mg/mL) is a clear, colorless, sterile solution. It is supplied in single-dose 5 mL vials (NDC 43547-454-41), available in cartons of 10 vials (NDC 43547-454-10).
Close16.2 Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15oC to 30oC (59oF to 86oF) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION Psychiatric Reactions and Changes in Behavior - Advise patients and their caregivers that levetiracetam may cause changes in behavior (e.g., aggression, agitation, anger, anxiety, apathy ...
Psychiatric Reactions and Changes in Behavior
Advise patients and their caregivers that levetiracetam may cause changes in behavior (e.g., aggression, agitation, anger, anxiety, apathy, depression, hostility, and irritability) and psychotic symptoms [see Warnings and Precautions (5.1)].
Effects on Driving or Operating Machinery
Inform patients that levetiracetam may cause dizziness and somnolence. Inform patients not to drive or operate machinery until they have gained sufficient experience on levetiracetam to gauge whether it adversely affects their ability to drive or operate machinery [see Warnings and Precautions (5.2)].
Anaphylaxis and Angioedema
Advise patients to discontinue levetiracetam and seek medical care if they develop signs and symptoms of anaphylaxis or angioedema [see Warnings and Precautions (5.3)].
Dermatological Adverse Reactions
Advise patients that serious dermatological adverse reactions have occurred in patients treated with levetiracetam and instruct them to call their physician immediately if a rash develops [see Warnings and Precautions (5.4)].
DRESS/Multiorgan Hypersensitivity
Instruct patients and caregivers that a fever or rash associated with signs of other organ system involvement (e.g., lymphadenopathy, hepatic dysfunction) may be drug-related and should be reported to their healthcare provider immediately. Levetiracetam should be discontinued immediately if a serious hypersensitivity reaction is suspected [see Warnings and Precautions (5.5)].
Withdrawal of Levetiracetam
Advise patients and caregivers not to discontinue use of levetiracetam without consulting with their healthcare provider. Levetiracetam should normally be gradually withdrawn to reduce the potential of increased seizure frequency and status epilepticus [see Warnings and Precautions (5.7)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during levetiracetam therapy. Encourage patients to enroll in the North American Antiepileptic Drug (NAAED) pregnancy registry if they become pregnant [see Use in Specific Populations (8.1)].
Distributed by: Solco Healthcare US, LLC
Somerset, NJ 08873, USA
Manufactured by: Zhejiang Huahai Pharmaceutical Co., Ltd.
Xunqiao, Linhai, Zhejiang 317024, China
Revised: 03/2025
203404-03
Close -
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Carton Label - NDC 43547-454-10 - Levetiracetam Injection, USP - 500 mg/5 mL (100 mg/mL) Rx only - Description - Each vial contains 500 mg levetiracetam, 8.2 mg sodium acetate trihydrate, 45 mg ...
Carton Label
NDC 43547-454-10
Levetiracetam Injection, USP
500 mg/5 mL (100 mg/mL)
Rx only
Description
Each vial contains 500 mg levetiracetam, 8.2 mg sodium acetate trihydrate, 45 mg sodium chloride, glacial acetic acid for pH adjustment.
Usual Dosage
For intravenous use only. Must be diluted prior to administration. Single dose vials. Discard unused portion. See package insert for complete dosage recommendations
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15oC-30oC (59oF-86oF) [see USP Controlled Room Temperature].
Manufactured by
Zhejiang Huahai Pharmaceutical Co., Ltd
Xunqiao, Linhai, Zhejiang 317024, China
Distributed by:
Solco Healthcare US, LLC
Somerset, NJ 08873, USA
Rev: 12/2024
203310-02
Close -
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Vial Label - NDC 43547-454-41 - Levetiracetam Injection, USP - 500 mg/5 mL (100 mg/mL) Rx only - 5 mL single-dose vial - Usual Dosage: See package insert. Storage: Store at 20°C to 25°C (68°F to ...
Vial Label
NDC 43547-454-41
Levetiracetam Injection, USP
500 mg/5 mL (100 mg/mL)
Rx only
5 mL single-dose vial
Usual Dosage: See package insert.
Storage: Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15oC-30oC (59oF-86oF) [see USP Controlled Room Temperature].
FOR INTRAVENOUS USE ONLY.
MUST BE DILUTED PRIOR TO ADMINISTRATION.
Manufactured by:
Zhejiang Huahai Pharmaceutical Co., Ltd.
Xunqiao Town, Linhai, Zhejiang 317024, China
Distributed by:
Solco Healthcare US, LLC
Somerset, NJ 08873, USA
Rev: 03/2022
203309-01
Close -
INGREDIENTS AND APPEARANCEProduct Information
LEVETIRACETAM levetiracetam injection Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:43547-454 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVETIRACETAM (UNII: 44YRR34555) (LEVETIRACETAM - UNII:44YRR34555) LEVETIRACETAM 500 mg in 5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM ACETATE (UNII: 4550K0SC9B) ACETIC ACID (UNII: Q40Q9N063P) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43547-454-10 10 in 1 CARTON 04/11/2022 1 NDC:43547-454-41 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209474 04/11/2022 Labeler - Solco Healthcare US, LLC (828343017) Registrant - Prinston Pharmaceutical Inc. (967289799)
CloseEstablishment Name Address ID/FEI Business Operations Zhejiang Huahai Pharmaceutical Co., LTD 530732460 MANUFACTURE(43547-454)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
LEVETIRACETAM injection
Number of versions: 2
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Jun 2, 2025 | 3 (current) | download |
| May 5, 2022 | 1 | download |
Get Label RSS Feed for this Drug
LEVETIRACETAM injection
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=cfe618ae-f7de-43d8-a754-3ce0a08c48ee
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
LEVETIRACETAM injection
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 43547-454-10 |
| 2 | 43547-454-41 |