Label: HAND WASH- benzalkonium chloride liquid
- NDC Code(s): 64024-466-96
- Packager: Aldi, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- purpose
- Use
- warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
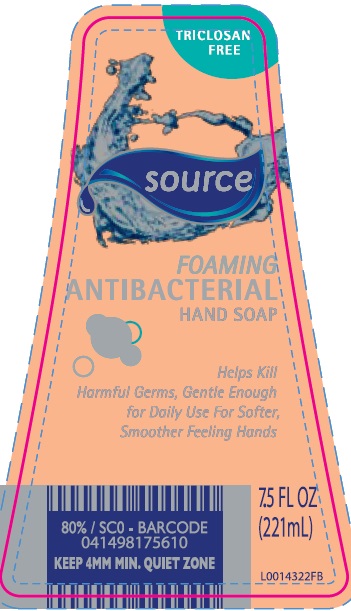
- principal display panel
-
INGREDIENTS AND APPEARANCE
HAND WASH
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64024-466 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) LAURAMIDOPROPYLAMINE OXIDE (UNII: I6KX160QTV) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) MYRISTAMIDOPROPYLAMINE OXIDE (UNII: 3HSF539C9T) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE SODIUM (UNII: MP1J8420LU) SULISOBENZONE (UNII: 1W6L629B4K) SODIUM BENZOATE (UNII: OJ245FE5EU) FD&C RED NO. 4 (UNII: X3W0AM1JLX) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64024-466-96 221 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/17/2016 08/14/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 02/17/2016 08/14/2019 Labeler - Aldi, Inc (944259522) Registrant - Consumer Product Partners, LLC (119091520) Establishment Name Address ID/FEI Business Operations Consumer Product Partners, LLC 119091520 manufacture(64024-466) Establishment Name Address ID/FEI Business Operations Consumer Product Partners, LLC 119091514 manufacture(64024-466)