Label: FENTANYL CITRATE injection
- NDC Code(s): 0641-6024-01, 0641-6024-10, 0641-6025-01, 0641-6025-10, view more
- Packager: Hikma Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: New Drug Application
Drug Label Information
Updated March 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FENTANYL CITRATE INJECTION safely and effectively. See full prescribing information for FENTANYL CITRATE INJECTION. Fentanyl ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS AND LIFE-THREATENING RISKS FROM USE OF FENTANYL CITRATE INJECTION
Addiction, Abuse, and Misuse
Because the use of Fentanyl Citrate Injection exposes patients and other users to the risks of opioid addiction, abuse and misuse, which can lead to overdose and death, assess each patient’s risk prior to prescribing and reassess all patients regularly for the development of these behaviors and conditions [see Warnings and Precautions (5.1)].
Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression may occur with use of Fentanyl Citrate Injection, especially during initiation or following a dosage increase. To reduce the risk of respiratory depression, proper dosing and titration of Fentanyl Citrate Injection are essential [see Warnings and Precautions (5.2)].
Risks From Concomitant Use With Benzodiazepines Or Other CNS Depressants
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of Fentanyl Citrate Injection and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate [see Warnings and Precautions (5.4), Drug Interactions (7)].
Close
Cytochrome P450 3A4 Interaction
The concomitant use of Fentanyl Citrate Injection with all cytochrome P450 3A4 inhibitors may result in an increase in fentanyl plasma concentrations, which could increase or prolong adverse reactions and may cause potentially fatal respiratory depression. In addition, discontinuation of a concomitantly used cytochrome P450 3A4 inducer may result in an increase in fentanyl plasma concentration. Monitor patients receiving Fentanyl Citrate Injection and any CYP3A4 inhibitor or inducer [see Warnings and Precautions (5.3), Drug Interactions (7), Clinical Pharmacology (12.3)] -
1 INDICATIONS AND USAGE
Fentanyl Citrate Injection is indicated for: analgesic action of short duration during the anesthetic periods, premedication, induction and maintenance, and in the immediate postoperative period ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions - Fentanyl Citrate Injection should be administered only by persons specifically trained in the use of intravenous anesthetics and ...
-
3 DOSAGE FORMS AND STRENGTHS
Single-Dose Ampuls, Vials and Prefilled Syringes: Fentanyl Citrate Injection, USP, equivalent to 50 mcg fentanyl base per mL, is a preservative-free solution, available in 2 mL, 5 mL, 20 mL ...
-
4 CONTRAINDICATIONS
Fentanyl Citrate Injection is contraindicated in patients with: Hypersensitivity to fentanyl (e.g., anaphylaxis) [See Adverse Reactions (6)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Addiction, Abuse, and Misuse - Fentanyl Citrate Injection contains fentanyl, a Schedule II controlled substance. As an opioid, Fentanyl Citrate Injection exposes users to the risks of ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described, or described in greater detail, in other sections: Addiction, Abuse, and Misuse [see Warnings and Precautions (5.1)] Life-Threatening ...
-
7 DRUG INTERACTIONS
Table 2 includes clinically significant drug interactions with Fentanyl Citrate Injection. Table 2: Clinically Significant Drug Interactions with Fentanyl Citrate Injection - Inhibitors of ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Use of opioid analgesics for an extended period of time during pregnancy may cause neonatal opioid withdrawal syndrome. Available data with Fentanyl Citrate ...
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance - Fentanyl Citrate Injection contains fentanyl, a Schedule II controlled drug substance. 9.2 Abuse - Fentanyl Citrate Injection contains fentanyl, a substance with ...
-
10 OVERDOSAGE
Clinical Presentation - Acute overdose with fentanyl can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin ...
-
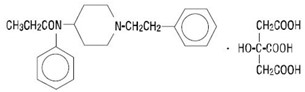
11 DESCRIPTION
Fentanyl Citrate Injection is an opioid agonist, available as a sterile, non-pyrogenic solution containing fentanyl citrate as the active pharmaceutical ingredient, for intravenous or ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Fentanyl Citrate Injection is an opioid agonist, whose principal actions of therapeutic value are analgesia and sedation. 12.2 Pharmacodynamics - Effects on the ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies in animals to evaluate the carcinogenic potential of fentanyl citrate have not been conducted ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Fentanyl Citrate Injection, equivalent to 50 mcg fentanyl base per mL, is a preservative-free solution, supplied as follows: NDC 0641-6024-10 2 mL Single Dose ampuls packaged in 10s ...
-
17 PATIENT COUNSELING INFORMATION
Addiction, Abuse, and Misuse - Inform patients that the use of Fentanyl Citrate Injection, even when taken as recommended, can result in addiction, abuse, and misuse, which can lead to overdose ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Hikma Pharmaceuticals USA Inc. Berkeley Heights, NJ 07922 - Revised: December 2023 - 462-254-12
-
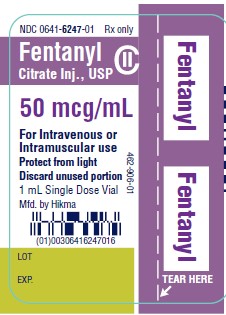
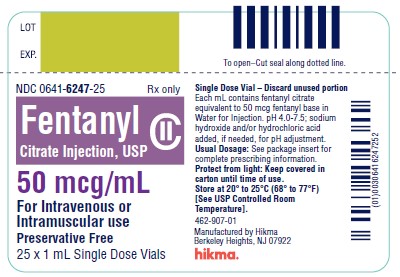
PRINCIPAL DISPLAY PANEL
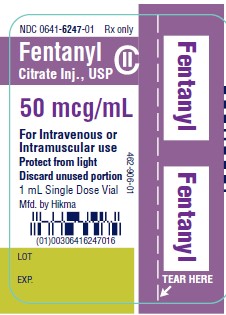
NDC 0641-6247-01 Rx only - Fentanyl - Citrate Inj., USP CII - 50 mcg/mL - For Intravenous or - Intramuscular use - Protect from light - Discard unused portion - 1 mL Single Dose ...
-
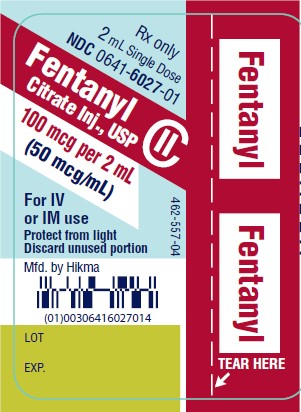
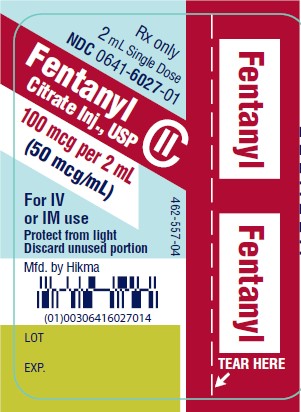
PRINCIPAL DISPLAY PANELRx only - 2 mL Single Dose - NDC 0641-6027-01 - Fentanyl Citrate Inj., USP CII - 100 mcg per 2 mL - (50 mcg/mL) For IV - or IM use - Protect from light - Discard unused portion - Rx only - 2 mL ...
-
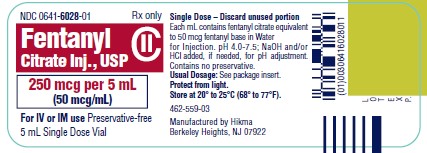
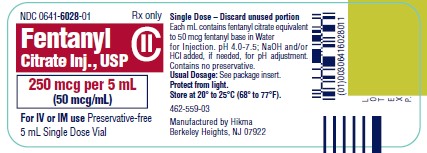
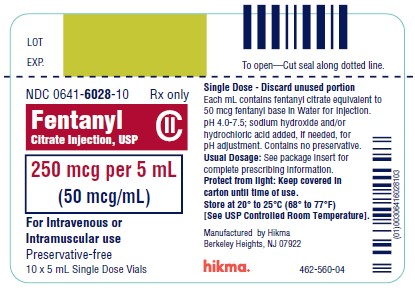
PRINCIPAL DISPLAY PANEL
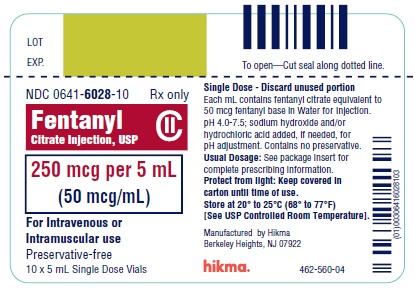
NDC 0641-6028-01 Rx only - Fentanyl Citrate Inj., USP CII - 250 mcg per 5 mL - (50 mcg/mL) For IV or IM use Preservative-free - 5 mL Single Dose Vial - NDC 0641-6028-10 Rx ...
-
PRINCIPAL DISPLAY PANEL
NDC 0641-6029-01 Rx only - Fentanyl Citrate Injection, USP CII - 1,000 mcg per 20 mL - (50 mcg/mL) For slow Intravenous use by hospital personnel - specifically trained in the use of narcotic ...
-
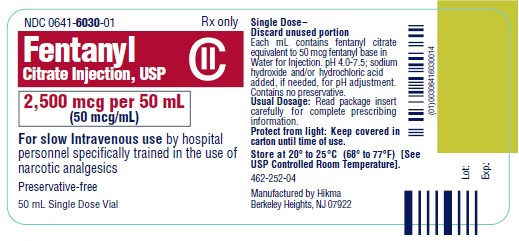
PRINCIPAL DISPLAY PANEL
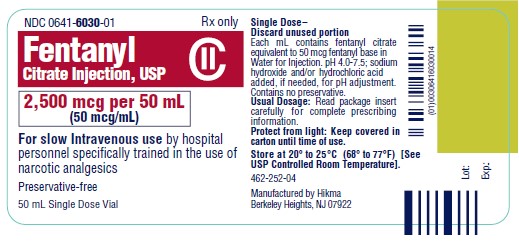
NDC 0641-6030-01 Rx only - Fentanyl Citrate Injection, USP CII - 2,500 mcg per 50 mL - (50 mcg/mL) For slow Intravenous use by hospital - personnel specifically trained in the use ...
-
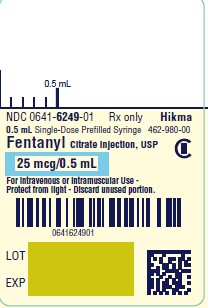
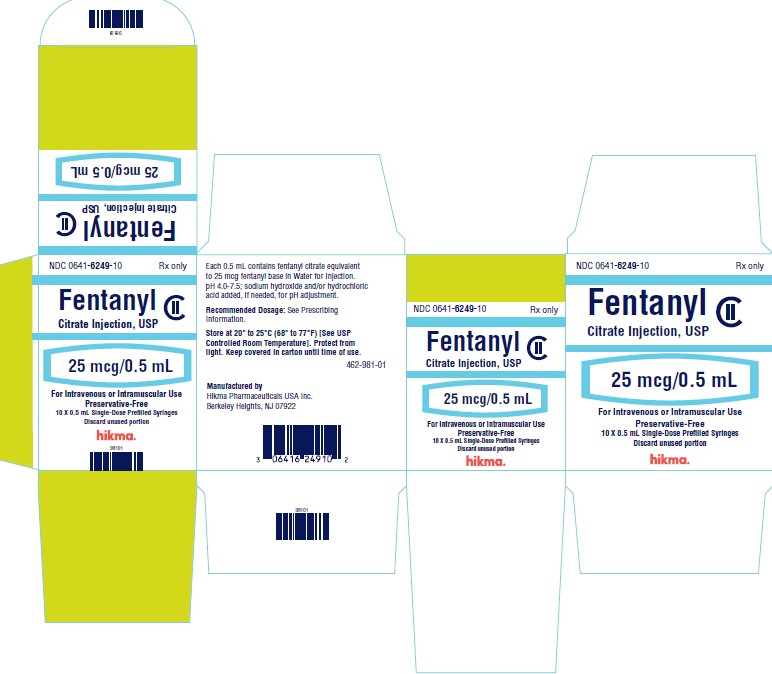
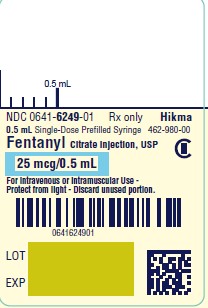
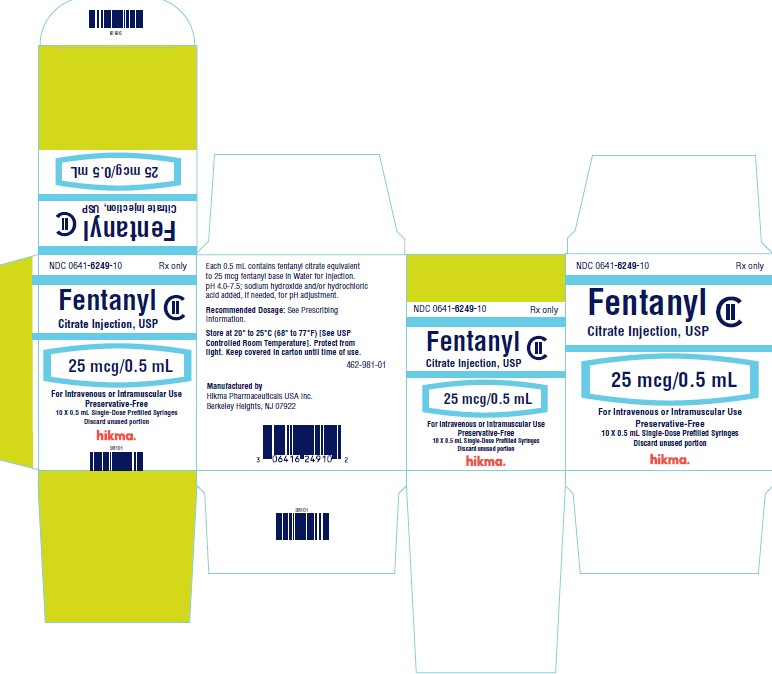
PRINCIPAL DISPLAY PANELNDC 0641-6249-01 Rx only - Fentanyl Citrate Injection, USP CII - 25 mcg/ 0.5 mL - For Intravenous or Intramuscular use - Preservative-free - 0.5 mL Single Dose Prefilled Syringe - NDC ...
-
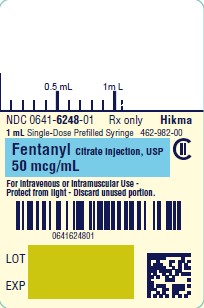
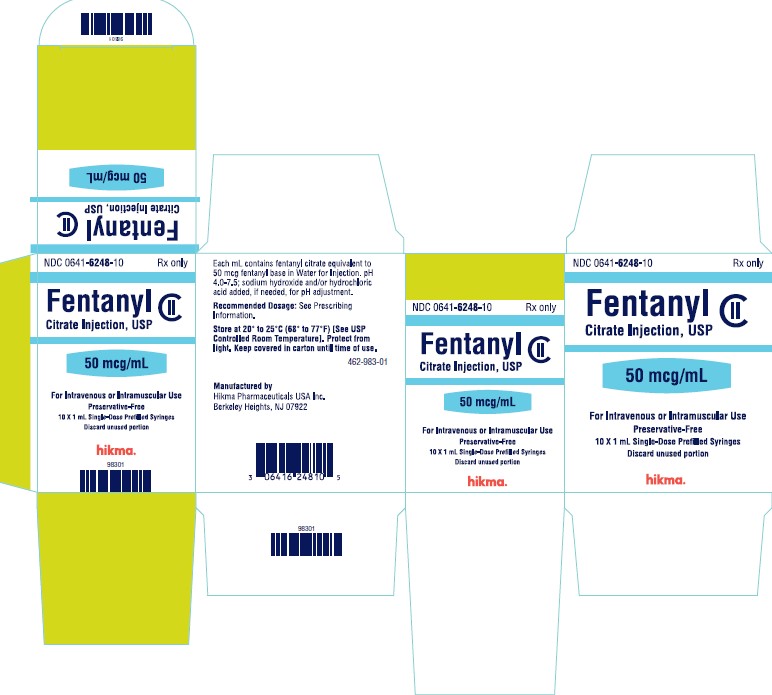
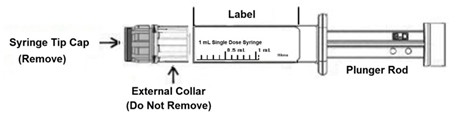
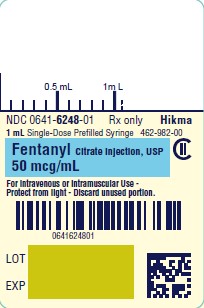
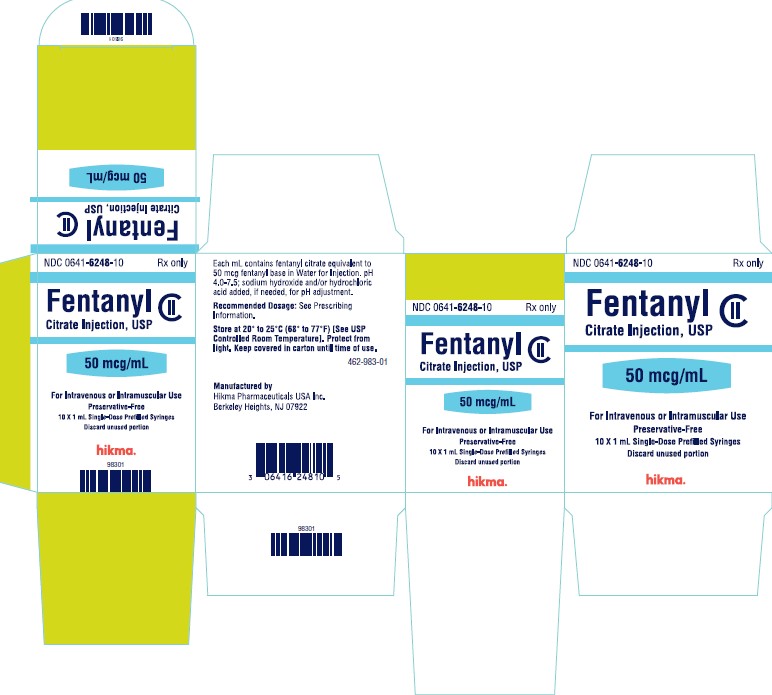
PRINCIPAL DISPLAY PANELNDC 0641-6248-01 Rx only - Fentanyl Citrate Injection, USP CII - 50 mcg/mL - For Intravenous or Intramuscular use - Preservative-free - 1 mL Single Dose Prefilled Syringe - NDC 0641-6248-10 ...
-
INGREDIENTS AND APPEARANCEProduct Information