Label: NITROFURANTOIN MACROCRYSTALS capsule
- NDC Code(s): 60687-839-01, 60687-839-11, 60687-850-01, 60687-850-11
- Packager: American Health Packaging
- This is a repackaged label.
- Source NDC Code(s): 68001-604, 68001-605
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
8483901/0324FTo reduce the development of drug-resistant bacteria and maintain the effectiveness of nitrofurantoin capsules (macrocrystals) and other antibacterial drugs, nitrofurantoin capsules ...
-
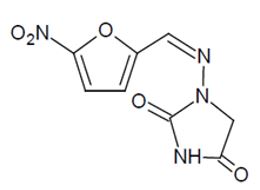
DESCRIPTIONNitrofurantoin capsules, USP (macrocrystals) are a synthetic chemical of controlled crystal size. It is a stable, lemon yellow, crystalline compound. Nitrofurantoin capsules, USP (macrocrystals ...
-
CLINICAL PHARMACOLOGYNitrofurantoin capsules (macrocrystals) is a larger crystal form of Furadantin - ® (nitrofurantoin). The absorption of nitrofurantoin capsules (macrocrystals) is slower and its excretion somewhat ...
-
MICROBIOLOGYNitrofurantoin is a nitrofuran antimicrobial agent with activity against certain Gram-positive and Gram-negative bacteria. Mechanism of Action - The mechanism of the antimicrobial action of ...
-
INDICATIONS AND USAGENitrofurantoin capsules (macrocrystals) are specifically indicated for the treatment of urinary tract infections when due to susceptible strains of - Escherichia coli, enterococci ...
-
CONTRAINDICATIONSAnuria, oliguria, or significant impairment of renal function (creatinine clearance under 60 mL per minute or clinically significant elevated serum creatinine) are contraindications. Treatment of ...
-
WARNINGSPulmonary reactions: ACUTE, SUBACUTE, OR CHRONIC PULMONARY REACTIONS HAVE BEEN OBSERVED IN PATIENTS TREATED WITH NITROFURANTOIN. IF THESE REACTIONS OCCUR, NITROFURANTOIN CAPSULES ...
-
PRECAUTIONSInformation for Patients: Patients should be advised to take nitrofurantoin capsules (macrocrystals) with food to further enhance tolerance and improve drug absorption. Patients should be ...
-
ADVERSE REACTIONSRespiratory: CHRONIC, SUBACUTE, OR ACUTE PULMONARY HYPERSENSITIVITY REACTIONS MAY OCCUR. CHRONIC PULMONARY REACTIONS OCCUR GENERALLY IN PATIENTS WHO HAVE RECEIVED CONTINUOUS TREATMENT ...
-
OVERDOSAGEOccasional incidents of acute overdosage of nitrofurantoin capsules (macrocrystals) have not resulted in any specific symptoms other than vomiting. Induction of emesis is recommended. There is no ...
-
DOSAGE AND ADMINISTRATIONNitrofurantoin capsules, (macrocrystals) should be given with food to improve drug absorption and, in some patients, tolerance. Adults: 50 mg to 100 mg four times a day - the lower dosage level ...
-
HOW SUPPLIEDNitrofurantoin capsules, USP (macrocrystals) are available as follows: 50 mg blue opaque cap and white opaque body, hard gelatin size "3" capsules imprinted with "LS" on cap and "411" on body ...
-
PACKAGING INFORMATIONAmerican Health Packaging unit dose blisters (see - How Supplied section) contain drug product from BluePoint Laboratories as follows: (50 mg / 100 UD) NDC 60687-839-01 packaged from NDC ...
-
Package/Label Display Panel – Carton – 50 mgNDC 60687- 839-01 - Nitrofurantoin - Capsules, USP - (Macrocrystals) 50 mg - 100 Capsules (10 x 10) Rx Only - URINARY TRACT ANTIBACTERIAL - Each Capsule Contains ...
-
Package/Label Display Panel – Blister – 50 mgNitrofurantoin - Capsule, USP - (Macrocrystals) 50 mg
-
Package/Label Display Panel – Carton – 100 mgNDC 60687- 850-01 - Nitrofurantoin - Capsules, USP - (Macrocrystals) 100 mg - 100 Capsules (10 x 10) Rx Only - URINARY TRACT ANTIBACTERIAL - Each Capsule Contains ...
-
Package/Label Display Panel – Blister – 100 mgNitrofurantoin - Capsule, USP - (Macrocrystals) 100 mg
-
INGREDIENTS AND APPEARANCEProduct Information