Label: AZACITIDINE injection, powder, lyophilized, for solution
- NDC Code(s): 68001-620-54
- Packager: BluePoint Laboratories.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 6, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AZACITIDINE FOR INJECTION safely and effectively. See full prescribing information for AZACITIDINE FOR INJECTION. AZACITIDINE for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Myelodysplastic Syndromes (MDS) Azacitidine for injection is indicated for treatment of adult patients with the following French-American ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Information - Do not substitute azacitidine for injection for oral azacitidine. The indications and dosing regimen for azacitidine for injection differ from that of ...
-
3 DOSAGE FORMS AND STRENGTHSAzacitidine for injection is supplied as lyophilized powder in 100 mg single-dose vials
-
4 CONTRAINDICATIONS4.1 Advanced Malignant Hepatic Tumors - Azacitidine for injection is contraindicated in patients with advanced malignant hepatic tumors [see Warnings and Precautions ( 5.3)]. 4.2 ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risks of Substitution with Other Azacitidine Products - Due to substantial differences in the pharmacokinetic parameters [see Clinical Pharmacology ( 12.3)], the recommended dose and ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described in other labelingsections: Anemia, Neutropenia and Thrombocytopenia [see Warnings and Precautions ( 5.2) ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action and findings in animals, azacitidine for injection can cause fetal harm when ...
-
10 OVERDOSAGEOne case of overdose with azacitidine for injection was reported during clinical trials. A patient experienced diarrhea, nausea, and vomiting after receiving a single intravenous dose of ...
-
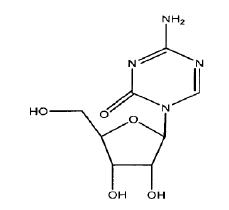
11 DESCRIPTIONAzacitidine for injection contains azacitidine, which is a nucleoside metabolic inhibitor. Azacitidine is 4-amino-1-β-D-ribofuranosyl-1,3,5-triazin-2(1H)-one. The structural formula is as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Azacitidine for injection is a pyrimidine nucleoside analog of cytidine. Azacitidine for injection is believed to exert its antineoplastic effects by causing ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - The potential carcinogenicity of azacitidine was evaluated in mice and rats. Azacitidine induced tumors of the hematopoietic system in ...
-
14 CLINICAL STUDIES14.1 Myelodysplastic Syndromes (MDS) Study 1 was a randomized, open-label, controlled trial carried out in 53 U.S. sites compared the safety and efficacy of subcutaneous azacitidine for ...
-
15 REFERENCES1. “OSHA Hazardous Drugs.” OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Azacitidine for injection is supplied as a lyophilized powder in 100 mg single-dose vials packaged in cartons of 1 vial (NDC 68001-620-54). Storage - Store unreconstituted vials at ...
-
17 PATIENT COUNSELING INFORMATIONHepatotoxicity in Patients with Severe Pre-Existing Hepatic Impairment - Instruct patients to inform their physician about any underlying liver disease [see Warnings and Precautions ( 5.3)] ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTIONVial Label
-
PRINCIPAL DISPLAY PANELVial Carton
-
INGREDIENTS AND APPEARANCEProduct Information