Label: REPATHA- evolocumab injection, solution
REPATHA- evolocumab kit
-
NDC Code(s):
72511-393-01,
72511-393-02,
72511-501-01,
72511-750-01, view more72511-760-01, 72511-760-02, 72511-760-91, 72511-770-01, 72511-770-91
- Packager: Amgen USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use REPATHA® safely and effectively. See full prescribing information for REPATHA. REPATHA (evolocumab) injection, for subcutaneous ...These highlights do not include all the information needed to use REPATHA® safely and effectively. See full prescribing information for REPATHA.
REPATHA (evolocumab) injection, for subcutaneous use
Initial U.S. Approval: 2015RECENT MAJOR CHANGES
INDICATIONS AND USAGE
REPATHA is a PCSK9 (proprotein convertase subtilisin kexin type 9) inhibitor indicated:
- To reduce the risk of major adverse cardiovascular (CV) events (CV death, myocardial infarction, stroke, unstable angina requiring hospitalization, or coronary revascularization) in adults with established cardiovascular disease (1)
- as an adjunct to diet, alone or in combination with other low-density lipoprotein cholesterol (LDL-C)-lowering therapies, in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH), to reduce LDL-C (1)
- as an adjunct to diet and other LDL-C-lowering therapies in pediatric patients aged 10 years and older with HeFH, to reduce LDL-C (1)
- as an adjunct to other LDL-C-lowering therapies in adults and pediatric patients aged 10 years and older with homozygous familial hypercholesterolemia (HoFH), to reduce LDL-C (1)
DOSAGE AND ADMINISTRATION
In adults with established CVD or with primary hyperlipidemia:
- The recommended dosage of REPATHA is either 140 mg every 2 weeks OR 420 mg once monthly administered subcutaneously. (2.1)
- If switching dosage regimens, administer the first dose of the new regimen on the next scheduled date of the prior regimen. (2.1)
In pediatric patients aged 10 years and older with HeFH:
- The recommended dosage of REPATHA is either 140 mg every 2 weeks OR 420 mg once monthly administered subcutaneously. (2.1)
- If switching dosage regimens, administer the first dose of the new regimen on the next scheduled date of the prior regimen. (2.1)
In adults and pediatric patients aged 10 years and older with HoFH:
- The initial recommended dosage of REPATHA is 420 mg once monthly administered subcutaneously. (2.1)
- The dosage can be increased to 420 mg every 2 weeks if a clinically meaningful response is not achieved in 12 weeks. (2.1)
- Patients on lipid apheresis may initiate treatment with 420 mg every 2 weeks to correspond with their apheresis schedule. Administer REPATHA after the apheresis session is complete. (2.1)
- Assess LDL-C when clinically appropriate. The LDL-lowering effect of REPATHA may be measured as early as 4 weeks after initiation. (2.1)
- REPATHA is available as prefilled single-dose SureClick® autoinjectors and prefilled single-dose syringes that either contain dry natural rubber (a derivative of latex) in the needle cover or are not made with natural rubber latex. Consider prescribing a presentation of REPATHA that does not contain dry natural rubber for individuals that are sensitive to latex. (2.3, 16)
- Administer REPATHA subcutaneously into areas of the abdomen, thigh, or upper arm. Rotate injection sites for each administration. (2.3)
- See Full Prescribing Information for important administration instructions. (2.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
Patients with a history of a serious hypersensitivity reaction to evolocumab or any of the excipients in REPATHA. (4)
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions: Angioedema has occurred. If signs or symptoms of serious hypersensitivity reactions occur, discontinue treatment with REPATHA, treat according to the standard of care, and monitor until signs and symptoms resolve. (5.1)
ADVERSE REACTIONS
Common (> 5% of patients treated with REPATHA and more frequently than placebo) adverse reactions in adults with:
Primary hyperlipidemia: nasopharyngitis, upper respiratory tract infection, influenza, back pain, and injection site reactions. (6)
Established CVD: diabetes mellitus, nasopharyngitis and upper respiratory tract infection. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Amgen Medical Information at 1-800-77-AMGEN (1-800-772-6436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Missed Doses
2.3 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGEREPATHA is indicated: To reduce the risk of major adverse cardiovascular (CV) events (CV death, myocardial infarction, stroke, unstable angina requiring hospitalization, or coronary ...
REPATHA is indicated:
- To reduce the risk of major adverse cardiovascular (CV) events (CV death, myocardial infarction, stroke, unstable angina requiring hospitalization, or coronary revascularization) in adults with established cardiovascular disease
- As an adjunct to diet, alone or in combination with other low-density lipoprotein cholesterol (LDL-C)-lowering therapies, in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH), to reduce LDL-C
- As an adjunct to diet and other LDL-C-lowering therapies in pediatric patients aged 10 years and older with HeFH, to reduce LDL-C
- As an adjunct to other LDL-C-lowering therapies in adults and pediatric patients aged 10 years and older with homozygous familial hypercholesterolemia (HoFH), to reduce LDL-C
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - In adults with established cardiovascular disease or with primary hyperlipidemia: The recommended dosage of REPATHA is either 140 mg every 2 weeks OR 420 mg once ...
2.1 Recommended Dosage
- In adults with established cardiovascular disease or with primary hyperlipidemia:
- The recommended dosage of REPATHA is either 140 mg every 2 weeks OR 420 mg once monthly administered subcutaneously [see Dosage and Administration (2.3)].
- If switching dosage regimens, administer the first dose of the new regimen on the next scheduled date of the prior regimen.
- In pediatric patients aged 10 years and older with HeFH:
- The recommended dosage of REPATHA is either 140 mg every 2 weeks OR 420 mg once monthly administered subcutaneously [see Dosage and Administration (2.3)].
- If switching dosage regimens, administer the first dose of the new regimen on the next scheduled date of the prior regimen.
- In adults and pediatric patients aged 10 years and older with HoFH:
- The initial recommended dosage of REPATHA is 420 mg once monthly administered subcutaneously [see Dosage and Administration (2.3)].
- The dosage can be increased to 420 mg every 2 weeks if a clinically meaningful response is not achieved in 12 weeks.
- Patients on lipid apheresis may initiate treatment with 420 mg every 2 weeks to correspond with their apheresis schedule. Administer REPATHA after the apheresis session is complete.
- Assess LDL-C when clinically appropriate. The LDL-lowering effect of REPATHA may be measured as early as 4 weeks after initiation.
- When monitoring LDL-C for patients receiving REPATHA 420 mg once monthly, note that LDL-C can vary during the dosing interval in some patients; recommend measuring LDL-C just prior to the next scheduled dose [see Clinical Studies (14)].
2.2 Missed Doses
If a dose is missed:
- Within 7 days from the missed dose, instruct the patient to administer REPATHA and resume the patient's original schedule.
- More than 7 days after the missed dose:
- For an every 2-week dose, instruct the patient to wait until the next dose on the original schedule.
- For a once-monthly dose, instruct the patient to administer the dose and start a new schedule based on this date.
Close2.3 Important Administration Instructions
- REPATHA is available as prefilled single-dose SureClick® autoinjectors and prefilled single-dose syringes that either contain dry natural rubber (a derivative of latex) in the needle cover or are not made with natural rubber latex [see How Supplied/Storage and Handling (16)]. Consider prescribing a presentation of REPATHA that does not contain dry natural rubber for individuals that are sensitive to latex [see Warnings and Precautions (5.1)].
- Train patients and/or caregivers on how to prepare and administer REPATHA, according to the Instructions for Use and instruct them to read and follow the Instructions for Use each time they use REPATHA.
- Prior to use, allow REPATHA to warm to room temperature for at least 30 minutes for the prefilled single-dose SureClick® autoinjector or prefilled single-dose syringe and for at least 45 minutes for the on-body infusor with prefilled cartridge if REPATHA has been refrigerated [see How Supplied/Storage and Handling (16)].
- Visually inspect REPATHA prior to administration. REPATHA is a clear to opalescent, colorless to pale yellow solution. Do not use if the solution is cloudy, discolored, or contains particles.
- Administer REPATHA subcutaneously into areas of the abdomen, thigh, or upper arm that are not tender, bruised, red, or indurated. Avoid injecting into areas with scars or stretch marks. Rotate injection sites for each administration.
- The 420 mg dose of REPATHA can be administered:
- over 5 minutes by using the single-dose on-body infusor with prefilled cartridge, or
- by giving 3 injections consecutively within 30 minutes using the prefilled single-dose SureClick® autoinjector or prefilled single-dose syringe.
- In adults with established cardiovascular disease or with primary hyperlipidemia:
-
3 DOSAGE FORMS AND STRENGTHSREPATHA is a clear to opalescent, colorless to pale yellow solution available as follows: Injection: 140 mg/mL solution in a prefilled single-dose SureClick® autoinjector - Injection: 140 mg/mL ...
REPATHA is a clear to opalescent, colorless to pale yellow solution available as follows:
- Injection: 140 mg/mL solution in a prefilled single-dose SureClick® autoinjector
- Injection: 140 mg/mL solution in a prefilled single-dose syringe
- Injection: 420 mg/3.5 mL solution in a single-dose Pushtronex® system (on-body infusor with prefilled cartridge)
-
4 CONTRAINDICATIONSREPATHA is contraindicated in patients with a history of a serious hypersensitivity reaction to evolocumab or any of the excipients in REPATHA. Serious hypersensitivity reactions including ...
REPATHA is contraindicated in patients with a history of a serious hypersensitivity reaction to evolocumab or any of the excipients in REPATHA. Serious hypersensitivity reactions including angioedema have occurred in patients treated with REPATHA [see Warnings and Precautions (5.1)].
Close -
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Hypersensitivity reactions, including angioedema, have been reported in patients treated with REPATHA. If signs or symptoms of serious hypersensitivity reactions ...Close
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including angioedema, have been reported in patients treated with REPATHA. If signs or symptoms of serious hypersensitivity reactions occur, discontinue treatment with REPATHA, treat according to the standard of care, and monitor until signs and symptoms resolve. REPATHA is contraindicated in patients with a history of serious hypersensitivity reactions to evolocumab or any excipient in REPATHA [see Contraindications (4)].
The prefilled single-dose SureClick® autoinjector and prefilled single-dose syringe presentations of REPATHA that contain dry natural rubber (a derivative of latex) in the needle cover may cause an allergic reaction in individuals sensitive to latex. Instruct patients to inform their healthcare provider if they are sensitive to latex. Consider prescribing a presentation of REPATHA that does not contain dry natural rubber for individuals that are sensitive to latex [see How Supplied/Storage and Handling (16)].
-
6 ADVERSE REACTIONSThe following adverse reactions are also discussed in other sections of the label: Hypersensitivity Reactions [see Warnings and Precautions (5.1)] 6.1 Clinical Trials Experience - Because ...
The following adverse reactions are also discussed in other sections of the label:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse Reactions in Adults with Primary Hyperlipidemia
The data described below reflect exposure to REPATHA in 8 placebo-controlled trials that included 2651 patients treated with REPATHA, including 557 exposed for 6 months and 515 exposed for 1 year (median treatment duration of 12 weeks). The mean age of the population was 57 years, 49% of the population were women, 85% White, 6% Black, 8% Asians, and 2% other races.
Adverse Reactions in a 52-Week Controlled Trial
In a 52-week, double-blind, randomized, placebo-controlled trial, 599 patients received 420 mg of REPATHA subcutaneously once monthly [see Clinical Studies (14)]. The mean age was 56 years (range: 22 to 75 years), 23% were older than 65 years, 52% women, 80% White, 8% Black, 6% Asian; 6% identified as Hispanic ethnicity. Adverse reactions reported in at least 3% of REPATHA-treated patients, and more frequently than in placebo-treated patients are shown in Table 1. Adverse reactions led to discontinuation of treatment in 2.2% of REPATHA-treated patients and 1% of placebo-treated patients. The most common adverse reaction that led to REPATHA treatment discontinuation and occurred at a rate greater than placebo was myalgia (0.3% versus 0% for REPATHA and placebo, respectively).
Table 1. Adverse Reactions Occurring in ≥ 3% of REPATHA-treated Patients and More Frequently than with Placebo in a 52-Week Trial Placebo
(N = 302)
%REPATHA
(N = 599)
%- *
- includes erythema, pain, bruising
Nasopharyngitis 9.6 10.5 Upper respiratory tract infection 6.3 9.3 Influenza 6.3 7.5 Back pain 5.6 6.2 Injection site reactions* 5.0 5.7 Cough 3.6 4.5 Urinary tract infection 3.6 4.5 Sinusitis 3.0 4.2 Headache 3.6 4.0 Myalgia 3.0 4.0 Dizziness 2.6 3.7 Musculoskeletal pain 3.0 3.3 Hypertension 2.3 3.2 Diarrhea 2.6 3.0 Gastroenteritis 2.0 3.0 Adverse Reactions in Seven Pooled 12-Week Controlled Trials
In seven pooled 12-week, double-blind, randomized, placebo-controlled trials, 993 patients received 140 mg of REPATHA subcutaneously every 2 weeks and 1059 patients received 420 mg of REPATHA subcutaneously monthly. The mean age was 57 years (range: 18 to 80 years), 29% were older than 65 years, 49% women, 85% White, 5% Black, 9% Asian; 5% identified as Hispanic ethnicity. Adverse reactions reported in at least 1% of REPATHA-treated patients, and more frequently than in placebo-treated patients, are shown in Table 2.
Table 2. Adverse Reactions Occurring in ≥ 1% of REPATHA-treated Patients and More Frequently than with Placebo in Pooled 12-Week Trials Placebo
(N = 1224)
%REPATHA*
(N = 2052)
%- *
- 140 mg every 2 weeks and 420 mg once monthly combined
Nasopharyngitis 3.9 4.0 Back pain 2.2 2.3 Upper respiratory tract infection 2.0 2.1 Arthralgia 1.6 1.8 Nausea 1.2 1.8 Fatigue 1.0 1.6 Muscle spasms 1.2 1.3 Urinary tract infection 1.2 1.3 Cough 0.7 1.2 Influenza 1.1 1.2 Contusion 0.5 1.0 Adverse Reactions in Eight Pooled Controlled Trials (Seven 12-Week Trials and One 52-Week Trial)
The adverse reactions described below are from a pool of the 52-week trial and seven 12-week trials. The mean and median exposure durations of REPATHA in this pool of eight trials were 20 weeks and 12 weeks, respectively.
Local Injection Site Reactions
Injection site reactions occurred in 3.2% and 3.0% of REPATHA-treated and placebo-treated patients, respectively. The most common injection site reactions were erythema, pain, and bruising. The proportions of patients who discontinued treatment due to local injection site reactions in REPATHA-treated patients and placebo-treated patients were 0.1% and 0%, respectively.
Hypersensitivity Reactions
Hypersensitivity reactions occurred in 5.1% and 4.7% of REPATHA-treated and placebo-treated patients, respectively. The most common hypersensitivity reactions were rash (1.0% versus 0.5% for REPATHA and placebo, respectively), eczema (0.4% versus 0.2%), erythema (0.4% versus 0.2%), and urticaria (0.4% versus 0.1%).
Adverse Reactions in the Cardiovascular Outcomes Trial
In a double-blind, randomized, placebo-controlled cardiovascular outcomes trial, 27,525 patients received at least one dose of REPATHA or placebo [see Clinical Studies (14)]. The mean age was 62.5 years (range: 40 to 86 years), 45% were 65 years or older, 9% were 75 years or older, 25% women, 85% White, 2% Black and 10% Asian; 8% identified as Hispanic ethnicity. Patients were exposed to REPATHA or placebo for a median of 24.8 months; 91% of patients were exposed for ≥ 12 months, 54% were exposed for ≥ 24 months and 5% were exposed for ≥ 36 months.
The safety profile of REPATHA in this trial was generally consistent with the safety profile described above in the 12- and 52-week controlled trials involving patients with primary hyperlipidemia. Common adverse reactions (> 5% of patients treated with REPATHA and occurring more frequently than placebo) included diabetes mellitus (8.8% REPATHA, 8.2% placebo), nasopharyngitis (7.8% REPATHA, 7.4% placebo), and upper respiratory tract infection (5.1% REPATHA, 4.8% placebo).
Among the 16,676 patients without diabetes mellitus at baseline, the incidence of new-onset diabetes mellitus during the trial was 8.1% in patients treated with REPATHA compared with 7.7% in patients that received placebo.
Adverse Reactions in Pediatric Patients with HeFH
In a 24-week, randomized, placebo-controlled, double-blind trial of 157 pediatric patients with HeFH, 104 patients received 420 mg REPATHA subcutaneously once monthly [see Clinical Studies (14)]. The mean age was 13.7 years (range: 10 to 17 years), 56% were female, 85% White, 1% Black, 1% Asian, and 13% other; 8% identified as Hispanic ethnicity. Common adverse reactions (> 5% of patients treated with REPATHA and occurring more frequently than placebo) included:
- Nasopharyngitis (12% versus 11%)
- Headache (11% versus 2%)
- Oropharyngeal pain (7% versus 0%)
- Influenza (6% versus 4%)
- Upper respiratory tract infection (6% versus 2%)
Adverse Reactions in Adults and Pediatric Patients with HoFH
In a 12-week, double-blind, randomized, placebo-controlled trial of 49 patients with HoFH, 33 patients received 420 mg of REPATHA subcutaneously once monthly [see Clinical Studies (14)]. The mean age was 31 years (range: 13 to 57 years), 49% were women, 90% White, 4% Asian, and 6% other. The adverse reactions that occurred in at least two (6.1%) REPATHA-treated patients, and more frequently than in placebo-treated patients, included:
- Upper respiratory tract infection (9.1% versus 6.3%)
- Influenza (9.1% versus 0%)
- Gastroenteritis (6.1% versus 0%)
- Nasopharyngitis (6.1% versus 0%)
In a multicenter, open-label 5-year extension study, 106 patients with HoFH, including 14 pediatric patients, received 420 mg of REPATHA subcutaneously once monthly or every 2 weeks [see Clinical Studies (14)]. The mean age was 34 years (range: 13 to 68 years), 51% were women, 80% White, 12% Asian, 1% Native American, and 7% other; 5% identified as Hispanic ethnicity. No new adverse reactions were observed during the open-label extension study.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to REPATHA in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
The immunogenicity of REPATHA has been evaluated using an electrochemiluminescent bridging screening immunoassay for the detection of binding anti-drug antibodies. For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies.
In a pool of placebo- and active-controlled clinical trials, 0.3% (48 out of 17,992) of adult patients treated with at least one dose of REPATHA tested positive for the development of binding antibodies. Patients whose sera tested positive for binding antibodies were further evaluated for neutralizing antibodies; none of the patients tested positive for neutralizing antibodies.
The development of anti-evolocumab antibodies was not detected in clinical trials of pediatric patients treated with REPATHA.
There was no evidence that the presence of anti-drug binding antibodies impacted the pharmacokinetic profile, clinical response, or safety of REPATHA.
Close6.3 Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of REPATHA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hypersensitivity reactions: Angioedema
- Influenza-like illness
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from clinical trials and postmarketing reports on REPATHA use in pregnant women are insufficient to evaluate for a drug-associated risk of major ...
8.1 Pregnancy
Risk Summary
Available data from clinical trials and postmarketing reports on REPATHA use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. In animal reproduction studies, there were no effects on pregnancy or neonatal/infant development when monkeys were subcutaneously administered evolocumab from organogenesis through parturition at dose exposures up to 12 times the exposure at the maximum recommended human dose of 420 mg every month. In a similar study with another drug in the PCSK9 inhibitor antibody class, humoral immune suppression was observed in infant monkeys exposed to that drug in utero at all doses. The exposures where immune suppression occurred in infant monkeys were greater than those expected clinically. No assessment for immune suppression was conducted with evolocumab in infant monkeys. Measurable evolocumab serum concentrations were observed in the infant monkeys at birth at comparable levels to maternal serum, indicating that evolocumab, like other IgG antibodies, crosses the placental barrier. Monoclonal antibodies are transported across the placenta in increasing amounts especially near term; therefore, evolocumab has the potential to be transmitted from the mother to the developing fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
There is a pregnancy safety study for REPATHA. If REPATHA is administered during pregnancy, healthcare providers should report REPATHA exposure by contacting Amgen at 1-800-77-AMGEN (1-800-772-6436) or https://wwwext.amgen.com/products/global-patient-safety/adverse-event-reporting.
Data
Animal Data
In cynomolgus monkeys, no effects on embryo-fetal or postnatal development (up to 6 months of age) were observed when evolocumab was dosed during organogenesis to parturition at 50 mg/kg once every 2 weeks by the subcutaneous route at exposures 30- and 12-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC. No test of humoral immunity in infant monkeys was conducted with evolocumab.
8.2 Lactation
Risk Summary
There is no information regarding the presence of evolocumab in human milk, the effects on the breastfed infant, or the effects on milk production. Human IgG is present in human milk, but published data suggest that breast milk antibodies do not enter the neonatal and infant circulation in substantial amounts.
The development and health benefits of breastfeeding should be considered along with the mother's clinical need for REPATHA and any potential adverse effects on the breastfed infant from REPATHA or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of REPATHA in combination with diet and other LDL-C-lowering therapies for the treatment of HoFH have been established in pediatric patients aged 10 years and older. Use of REPATHA for this indication is supported by evidence from an adequate and well-controlled trial in adults and pediatric patients aged 13 years and older with HoFH (including 7 pediatric patients treated with REPATHA) and from open-label studies which included an additional 19 pediatric patients aged 11 years and older with HoFH not previously treated with REPATHA [see Adverse Reactions (6.1) and Clinical Studies (14)].
The safety and effectiveness of REPATHA as an adjunct to diet and other LDL-C-lowering therapies for the treatment of HeFH have been established in pediatric patients aged 10 years and older. Use of REPATHA for this indication is based on data from a 24-week, randomized, placebo-controlled, double-blind trial in pediatric patients with HeFH. In the trial, 104 patients received REPATHA 420 mg subcutaneously once monthly and 53 patients received placebo; 39 patients (25%) were 10 to 11 years of age [see Adverse Reactions (6.1) and Clinical Studies (14)].
The safety and effectiveness of REPATHA have not been established in pediatric patients with HeFH or HoFH who are younger than 10 years old or in pediatric patients with other types of hyperlipidemia.
8.5 Geriatric Use
In controlled trials, 7656 (41%) patients treated with REPATHA were ≥ 65 years old and 1500 (8%) were ≥ 75 years old. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
No dose adjustment is needed in patients with renal impairment [see Clinical Pharmacology (12.3)].
Close8.7 Hepatic Impairment
No dose adjustment is needed in patients with mild to moderate hepatic impairment (Child-Pugh A or B). No data are available in patients with severe hepatic impairment [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTIONEvolocumab is a human monoclonal immunoglobulin G2 (IgG2) directed against human proprotein convertase subtilisin kexin type 9 (PCSK9). Evolocumab has an approximate molecular weight (MW) of 144 ...
Evolocumab is a human monoclonal immunoglobulin G2 (IgG2) directed against human proprotein convertase subtilisin kexin type 9 (PCSK9). Evolocumab has an approximate molecular weight (MW) of 144 kDa and is produced in genetically engineered mammalian (Chinese hamster ovary) cells.
REPATHA is a sterile, preservative-free, clear to opalescent, colorless to pale yellow solution for subcutaneous use. Each 1 mL prefilled single-dose SureClick® autoinjector and prefilled single-dose syringe contains 140 mg evolocumab, acetate (1.2 mg), polysorbate 80 (0.1 mg), proline (25 mg) in Water for Injection, USP. Sodium hydroxide may be used to adjust to a pH of 5.0. Each single-dose Pushtronex® system (on-body infusor with prefilled cartridge) delivers a 3.5 mL solution containing 420 mg evolocumab, acetate (4.2 mg), polysorbate 80 (0.35 mg), proline (89 mg) in Water for Injection, USP. Sodium hydroxide may be used to adjust to a pH of 5.0.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Evolocumab is a human monoclonal IgG2 directed against human proprotein convertase subtilisin kexin type 9 (PCSK9). PCSK9 binds to the low-density lipoprotein receptor ...
12.1 Mechanism of Action
Evolocumab is a human monoclonal IgG2 directed against human proprotein convertase subtilisin kexin type 9 (PCSK9). PCSK9 binds to the low-density lipoprotein receptor (LDLR) on the surface of hepatocytes to promote LDLR degradation within the liver. By inhibiting the binding of PCSK9 to LDLR, evolocumab increases the number of LDLRs available to clear LDL from the blood, thereby lowering LDL-C levels.
12.2 Pharmacodynamics
Following single subcutaneous administration of 140 mg or 420 mg of evolocumab, maximum suppression of circulating unbound PCSK9 occurred by 4 hours. Unbound PCSK9 concentrations returned toward baseline when evolocumab concentrations decreased below the limit of quantitation. Maximum LDL-C reduction occurred by 2 weeks after a single-dose of 140 mg of evolocumab and by 3 weeks after a single-dose of 420 mg of evolocumab.
Close12.3 Pharmacokinetics
Evolocumab exhibits non-linear kinetics as a result of binding to PCSK9. Administration of the 140 mg dose in healthy volunteers resulted in a Cmax mean of 18.6 μg/mL and AUClast mean of 188 day∙μg/mL. Administration of the 420 mg dose in healthy volunteers resulted in a Cmax mean of 59.0 μg/mL and AUClast mean of 924 day∙μg/mL. Following a single 420 mg intravenous dose, the mean systemic clearance was estimated to be 12 mL/hr. An approximate 2- to 3-fold accumulation was observed in trough serum concentrations (Cmin 7.21) following 140 mg doses administered subcutaneously every 2 weeks or following 420 mg doses administered subcutaneously monthly (Cmin 11.2), and serum trough concentrations approached steady-state by 12 weeks of dosing.
Absorption
Following a single subcutaneous dose of 140 mg or 420 mg evolocumab administered to healthy adults, median peak serum concentrations were attained in 3 to 4 days, and estimated absolute bioavailability was 72%.
Distribution
Following a single 420 mg intravenous dose, the mean steady-state volume of distribution was estimated to be 3.3 L.
Elimination
Two elimination phases were observed for REPATHA. At low concentrations, the elimination is predominately through saturable binding to target (PCSK9), while at higher concentrations the elimination of REPATHA is largely through a non-saturable proteolytic pathway. REPATHA was estimated to have an effective half-life of 11 to 17 days.
Specific Populations
The pharmacokinetics of evolocumab were not affected by age, gender, race, or creatinine clearance across all approved populations [see Use in Specific Populations (8.5)].
The exposure of evolocumab decreased with increasing body weight. These differences are not clinically meaningful.
Pediatric Patients
The pharmacokinetics of REPATHA were evaluated in 103 pediatric patients aged 10 to 17 years with HeFH (Study 6) [see Use in Specific Populations (8.4), and Clinical Studies (14)]. Following subcutaneous administration of 420 mg REPATHA once monthly, mean trough serum concentrations were 22.4 mcg/mL and 25.8 mcg/mL over the Week 12 and Week 24 time points, respectively. The pharmacokinetics of REPATHA were evaluated in 12 pediatric patients aged 11 to 17 years with HoFH (Study 9) [see Use in Specific Populations (8.4), and Clinical Studies (14)]. Following subcutaneous administration of 420 mg REPATHA once monthly, mean serum trough concentrations were 20.3 mcg/mL and 17.6 mcg/mL at Week 12 and Week 80, respectively.
Renal Impairment
Since monoclonal antibodies are not known to be eliminated via renal pathways, renal function is not expected to impact the pharmacokinetics of evolocumab.
In a clinical trial of 18 patients with either normal renal function (estimated glomerular filtration rate [eGFR] ≥ 90 mL/min/1.73 m2, n = 6), severe renal impairment (eGFR < 30 mL/min/1.73 m2, n = 6), or end-stage renal disease (ESRD) receiving hemodialysis (n = 6), exposure to evolocumab after a single 140 mg subcutaneous dose was decreased in patients with severe renal impairment or ESRD receiving hemodialysis. Reductions in PCSK9 levels in patients with severe renal impairment or ESRD receiving hemodialysis was similar to those with normal renal function [see Use in Specific Populations (8.6)].
Hepatic Impairment
Following a single 140 mg subcutaneous dose of evolocumab in patients with mild or moderate hepatic impairment, a 20-30% lower mean Cmax and 40-50% lower mean AUC were observed as compared to healthy patients [see Use in Specific Populations (8.7)].
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - The carcinogenic potential of evolocumab was evaluated in a lifetime study conducted in the hamster at dose levels of 10, 30, and 100 ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of evolocumab was evaluated in a lifetime study conducted in the hamster at dose levels of 10, 30, and 100 mg/kg administered every 2 weeks. There were no evolocumab-related tumors at the highest dose at systemic exposures up to 38- and 15-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC. The mutagenic potential of evolocumab has not been evaluated; however, monoclonal antibodies are not expected to alter DNA or chromosomes.
There were no adverse effects on fertility (including estrous cycling, sperm analysis, mating performance, and embryonic development) at the highest dose in a fertility and early embryonic developmental toxicology study in hamsters when evolocumab was subcutaneously administered at 10, 30, and 100 mg/kg every 2 weeks. The highest dose tested corresponds to systemic exposures up to 30- and 12-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC. In addition, there were no adverse evolocumab-related effects on surrogate markers of fertility (reproductive organ histopathology, menstrual cycling, or sperm parameters) in a 6-month chronic toxicology study in sexually mature monkeys subcutaneously administered evolocumab at 3, 30, and 300 mg/kg once weekly. The highest dose tested corresponds to 744- and 300-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC.
Close13.2 Animal Toxicology and/or Pharmacology
During a 3-month toxicology study of 10 and 100 mg/kg once every 2 weeks evolocumab in combination with 5 mg/kg once daily rosuvastatin in adult monkeys, there were no effects of evolocumab on the humoral immune response to keyhole limpet hemocyanin (KLH) after 1 to 2 months exposure. The highest dose tested corresponds to exposures 54- and 21-fold higher than the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC. Similarly, there were no effects of evolocumab on the humoral immune response to KLH (after 3 to 4 months exposure) in a 6-month study in cynomolgus monkeys at dose levels up to 300 mg/kg once weekly evolocumab corresponding to exposures 744- and 300-fold greater than the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC.
-
14 CLINICAL STUDIESAdult Patients with Established Cardiovascular Disease - Study 1 (FOURIER, NCT01764633) was a double-blind, randomized, placebo-controlled, event-driven trial in 27,564 (13,784 REPATHA, 13,780 ...
Adult Patients with Established Cardiovascular Disease
Study 1 (FOURIER, NCT01764633) was a double-blind, randomized, placebo-controlled, event-driven trial in 27,564 (13,784 REPATHA, 13,780 placebo) adult patients with established cardiovascular disease and with LDL-C ≥ 70 mg/dL and/or non-HDL-C ≥ 100 mg/dL despite high- or moderate-intensity statin therapy. Patients were randomly assigned 1:1 to receive either subcutaneous injections of REPATHA (140 mg every 2 weeks or 420 mg once monthly) or placebo; 86% used the every-2-week regimen throughout the trial. The median follow-up duration was 26 months. Overall, 99.2% of patients were followed until the end of the trial or death.
The mean (SD) age at baseline was 63 (9) years, with 45% being at least 65 years old; 25% were women. The trial population was 85% White, 2% Black, and 10% Asian; 8% identified as Hispanic ethnicity. Regarding prior diagnoses of cardiovascular disease, 81% had prior myocardial infarction, 19% prior non-hemorrhagic stroke, and 13% had symptomatic peripheral arterial disease. Selected additional baseline risk factors included hypertension (80%), diabetes mellitus (1% type 1; 36% type 2), current daily cigarette smoking (28%), New York Heart Association class I or II congestive heart failure (23%), and eGFR < 60 mL/min per 1.73 m2 (6%). Most patients were on a high- (69%) or moderate-intensity (30%) statin therapy at baseline, and 5% were also taking ezetimibe. Most patients were taking at least one other cardiovascular medication including anti-platelet agents (93%), beta blockers (76%), angiotensin converting enzyme (ACE) inhibitors (56%), or angiotensin receptor blockers (23%). On stable background lipid-lowering therapy, the median [Q1, Q3] LDL-C at baseline was 92 [80, 109] mg/dL; the mean (SD) was 98 (28) mg/dL.
REPATHA significantly reduced the risk for the primary composite endpoint (time to first occurrence of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization; p < 0.0001) and the key secondary composite endpoint (time to first occurrence of cardiovascular death, myocardial infarction, or stroke; p < 0.0001). The Kaplan-Meier estimates of the cumulative incidence of the primary and key secondary composite endpoints over time are shown in Figure 1 and Figure 2 below.
The results of primary and secondary efficacy endpoints are shown in Table 3 below.
Table 3. Effect of REPATHA on Cardiovascular Events in Patients with Established Cardiovascular Disease in FOURIER Placebo REPATHA REPATHA vs. Placebo N = 13780
n (%)Incidence Rate (per 100 patient years) N = 13784
n (%)Incidence Rate (per 100 patient years) Hazard Ratio
(95% CI)Primary composite endpoint Time to first occurrence of cardiovascular death, myocardial infarction, stroke, coronary revascularization, hospitalization for unstable angina 1563 (11.3) 5.2 1344 (9.8) 4.5 0.85
(0.79, 0.92)Key secondary composite endpoint Time to first occurrence of cardiovascular death, myocardial infarction, stroke 1013 (7.4) 3.4 816 (5.9) 2.7 0.80
(0.73, 0.88)Other secondary endpoints Time to cardiovascular death 240 (1.7) 0.8 251 (1.8) 0.8 1.05
(0.88, 1.25)Time to death by any cause* 426 (3.1) 1.4 444 (3.2) 1.5 1.04
(0.91, 1.19)Time to first fatal or non-fatal myocardial infarction 639 (4.6) 2.1 468 (3.4) 1.6 0.73
(0.65, 0.82)Time to first fatal or non-fatal stroke 262 (1.9) 0.9 207 (1.5) 0.7 0.79
(0.66, 0.95)Time to first coronary revascularization 965 (7.0) 3.2 759 (5.5) 2.5 0.78
(0.71, 0.86)Time to first hospitalization for unstable angina† 239 (1.7) 0.8 236 (1.7) 0.8 0.99
(0.82, 1.18)Figure 1. Estimated Cumulative Incidence of Primary Composite Endpoint Over 3 Years in FOURIER

Figure 2. Estimated Cumulative Incidence of Key Secondary Composite Endpoint Over 3 Years in FOURIER

The difference between REPATHA and placebo in mean percent change in LDL-C from baseline to Week 12 was −63% (95% CI: −63%, −62%) and from baseline to Week 72 was −57% (95% CI: −58%, −56%). At Week 48, the median [Q1, Q3] LDL-C was 26 [15, 46] mg/dL in the REPATHA group, with 47% of patients having LDL-C < 25 mg/dL.
In EBBINGHAUS (NCT02207634), a substudy of 1974 patients enrolled in the FOURIER trial, REPATHA was non-inferior to placebo on selected cognitive function domains as assessed with the use of neuropsychological function tests over a median follow-up of 19 months.
Primary Hyperlipidemia
Study 2 (LAPLACE-2, NCT01763866) was a multicenter, double-blind, randomized controlled 12-week trial in which patients were initially randomized to an open-label specific statin regimen for a 4-week lipid stabilization period followed by random assignment to subcutaneous injections of REPATHA 140 mg every 2 weeks, REPATHA 420 mg once monthly, or placebo for 12 weeks. The trial included 1896 patients with hyperlipidemia who received REPATHA, placebo, or ezetimibe as add-on therapy to daily doses of statins (atorvastatin, rosuvastatin, or simvastatin). Ezetimibe was also included as an active control only among those assigned to background atorvastatin. Overall, the mean age at baseline was 60 years (range: 20 to 80 years), 35% were ≥ 65 years old, 46% women, 94% White, 4% were Black, and 1% Asian; 5% identified as Hispanic or Latino ethnicity. After 4 weeks of background statin therapy, the mean baseline LDL-C ranged between 77 and 127 mg/dL across the five background therapy arms.
The difference between REPATHA and placebo in mean percent change in LDL-C from baseline to Week 12 was −71% (95% CI: −74%, −67%; p < 0.0001) and −63% (95% CI: −68%, −57%; p < 0.0001) for the 140 mg every 2 weeks and 420 mg once monthly dosages, respectively. The difference between REPATHA and ezetimibe in mean percent change in LDL-C from baseline to Week 12 was −45% (95% CI: −52%, −39%; p < 0.0001) and −41% (95% CI: −47%, −35%; p < 0.0001) for the 140 mg every 2 weeks and 420 mg once monthly dosages, respectively. For additional results, see Table 4 and Figure 3.
Table 4. Effect of REPATHA on Lipid Parameters in Patients with Hyperlipidemia on Background Statin Regimens (Mean % Change from Baseline to Week 12 in LAPLACE-2) Treatment Group LDL-C Non-HDL-C Apo B Total Cholesterol Estimates based on a multiple imputation model that accounts for treatment adherence - *
- 140 mg every 2 weeks or 420 mg once monthly yield similar reductions in LDL-C
REPATHA every 2 weeks vs. Placebo every 2 weeks
(Background statin: atorvastatin 10 mg or 80 mg; rosuvastatin 5 mg or 40 mg; simvastatin 40 mg)Placebo every 2 weeks (n = 281) 8 6 5 4 REPATHA 140 mg every 2 weeks* (n = 555) -63 -53 -49 -36 Mean difference from placebo
(95% CI)-71
(-74, -67)-59
(-62, -55)-55
(-58, -52)-40
(-43, -38)REPATHA once monthly vs. Placebo once monthly
(Background statin: atorvastatin 10 mg or 80 mg; rosuvastatin 5 mg or 40 mg; simvastatin 40 mg)Placebo once monthly (n = 277) 4 5 3 2 REPATHA 420 mg once monthly (n = 562) -59 -50 -46 -34 Mean difference from placebo
(95% CI)-63
(-68, -57)-54
(-58, -50)-50
(-53, -47)-36
(-39, -33)REPATHA every 2 weeks vs. Ezetimibe 10 mg daily
(Background statin: atorvastatin 10 mg or 80 mg)Ezetimibe 10 mg daily (n = 112) -17 -16 -14 -12 REPATHA 140 mg every 2 weeks* (n = 219) -63 -52 -49 -36 Mean difference from Ezetimibe
(95% CI)-45
(-52, -39)-36
(-41, -31)-35
(-40, -31)-24
(-28, -20)REPATHA once monthly vs. Ezetimibe 10 mg daily
(Background statin: atorvastatin 10 mg or 80 mg)Ezetimibe 10 mg daily (n = 109) -19 -16 -11 -12 REPATHA 420 mg once monthly (n = 220) -59 -50 -46 -34 Mean difference from Ezetimibe
(95% CI)-41
(-47, -35)-35
(-40, -29)-34
(-39, -30)-22
(-26, -19)Figure 3. Effect of REPATHA on LDL-C in Patients with Hyperlipidemia when Combined with Statins (Mean % Change from Baseline to Week 12 in LAPLACE-2)
Estimates based on a multiple imputation model that accounts for treatment adherence
Error bars indicate 95% confidence intervals
Study 3 (DESCARTES, NCT01516879) was a multicenter, double-blind, randomized, placebo-controlled, 52-week trial that included 901 patients with hyperlipidemia who received protocol-determined background lipid-lowering therapy of a cholesterol-lowering diet either alone or in addition to atorvastatin (10 mg or 80 mg daily) or the combination of atorvastatin 80 mg daily with ezetimibe. After stabilization on background therapy, patients were randomly assigned to the addition of placebo or REPATHA 420 mg administered subcutaneously once monthly. Overall, the mean age at baseline was 56 years (range: 25 to 75 years), 23% were ≥ 65 years, 52% women, 80% White, 8% Black, and 6% Asian; 6% identified as Hispanic or Latino ethnicity. After stabilization on the assigned background therapy, the mean baseline LDL-C ranged between 90 and 117 mg/dL across the four background therapy groups.
In these patients with hyperlipidemia on a protocol-determined background therapy, the difference between REPATHA 420 mg once monthly and placebo in mean percent change in LDL-C from baseline to Week 52 was −55% (95% CI: −60%, −50%; p < 0.0001) (Table 5 and Figure 4). For additional results, see Table 5.
Table 5. Effect of REPATHA on Lipid Parameters in Patients with Hyperlipidemia* (Mean % Change from Baseline to Week 52 in DESCARTES) Treatment Group LDL-C Non-HDL-C Apo B Total Cholesterol Estimates based on a multiple imputation model that accounts for treatment adherence - *
- Prior to randomization, patients were stabilized on background therapy consisting of a cholesterol-lowering diet either alone or in addition to atorvastatin (10 mg or 80 mg daily) or the combination of atorvastatin 80 mg daily with ezetimibe.
Placebo once monthly (n = 302) 8 8 2 5 REPATHA 420 mg once monthly (n = 599) -47 -39 -38 -26 Mean difference from placebo
(95% CI)-55
(-60, -50)-46
(-50, -42)-40
(-44, -37)-31
(-34, -28)Figure 4. Effect of REPATHA 420 mg Once Monthly on LDL-C in Patients with Hyperlipidemia in DESCARTES
Estimates based on a multiple imputation model that accounts for treatment adherence
Error bars indicate 95% confidence intervals
Study 4 (MENDEL-2, NCT01763827) was a multicenter, double-blind, randomized, placebo- and active-controlled, 12-week trial that included 614 patients with hyperlipidemia who were not taking lipid-lowering therapy at baseline. Patients were randomly assigned to receive subcutaneous injections of REPATHA 140 mg every 2 weeks, REPATHA 420 mg once monthly, or placebo for 12 weeks. Blinded administration of ezetimibe was also included as an active control. Overall, the mean age at baseline was 53 years (range: 20 to 80 years), 18% were ≥ 65 years old, 66% were women, 83% White, 7% Black, and 9% Asian; 11% identified as Hispanic or Latino ethnicity. The mean baseline LDL-C was 143 mg/dL.
The difference between REPATHA and placebo in mean percent change in LDL-C from baseline to Week 12 was −55% (95% CI: −60%, −50%; p < 0.0001) and −57% (95% CI: −61%, −52%; p < 0.0001) for the 140 mg every 2 weeks and 420 mg once monthly dosages, respectively. The difference between REPATHA and ezetimibe in mean percent change in LDL-C from baseline to Week 12 was −37% (95% CI: −42%, −32%; p < 0.0001) and −38% (95% CI: −42%, −34%; p < 0.0001) for the 140 mg every 2 weeks and 420 mg once monthly dosages, respectively. For additional results, see Table 6.
Table 6. Effect of REPATHA on Lipid Parameters in Patients with Hyperlipidemia (Mean % Change from Baseline to Week 12 in MENDEL-2) Treatment Group LDL-C Non-HDL-C Apo B Total Cholesterol Estimates based on a multiple imputation model that accounts for treatment adherence - *
- 140 mg every 2 weeks or 420 mg once monthly yield similar reductions in LDL-C
Placebo every 2 weeks (n = 76) 1 0 1 0 Ezetimibe 10 mg daily (n = 77) -17 -14 -13 -10 REPATHA 140 mg every 2 weeks* (n = 153) -54 -47 -44 -34 Mean difference from placebo
(95% CI)-55
(-60, -50)-47
(-52, -43)-45
(-50, -41)-34
(-37, -30)Mean difference from Ezetimibe
(95% CI)-37
(-42, -32)-33
(-37, -29)-32
(-36, -27)-23
(-27, -20)Placebo once monthly (n = 78) 1 2 2 0 Ezetimibe 10 mg daily (n = 77) -18 -16 -13 -12 REPATHA 420 mg once monthly (n = 153) -56 -49 -46 -35 Mean difference from placebo
(95% CI)-57
(-61, -52)-51
(-54, -47)-48
(-52, -44)-35
(-38, -32)Mean difference from Ezetimibe
(95% CI)-38
(-42, -34)-32
(-36, -29)-33
(-36, -29)-23
(-26, -20)Study 5 (RUTHERFORD-2, NCT01763918) was a multicenter, double-blind, randomized, placebo-controlled, 12-week trial in 329 patients with HeFH on statins with or without other lipid-lowering therapies. Patients were randomized to receive subcutaneous injections of REPATHA 140 mg every two weeks, 420 mg once monthly, or placebo. HeFH was diagnosed by the Simon Broome criteria (1991). In Study 5, 38% of patients had clinical atherosclerotic cardiovascular disease. The mean age at baseline was 51 years (range: 19 to 79 years), 15% of the patients were ≥ 65 years old, 42% were women, 90% were White, 5% were Asian, and 1% were Black. The average LDL-C at baseline was 156 mg/dL with 76% of the patients on high-intensity statin therapy.
The differences between REPATHA and placebo in mean percent change in LDL-C from baseline to Week 12 was −61% (95% CI: −67%, −55%; p < 0.0001) and −60% (95% CI: −68%, −52%; p < 0.0001) for the 140 mg every 2 weeks and 420 mg once monthly dosages, respectively. For additional results, see Table 7 and Figure 5.
Table 7. Effect of REPATHA on Lipid Parameters in Patients with HeFH (Mean % Change from Baseline to Week 12 in RUTHERFORD-2) Treatment Group LDL-C Non-HDL-C Apo B Total Cholesterol Estimates based on a multiple imputation model that accounts for treatment adherence - *
- 140 mg every 2 weeks or 420 mg once monthly yield similar reductions in LDL-C
Placebo every 2 weeks (n = 54) -1 -1 -1 -2 REPATHA 140 mg every 2 weeks* (n = 110) -62 -56 -49 -42 Mean difference from placebo
(95% CI)-61
(-67, -55)-54
(-60, -49)-49
(-54, -43)-40
(-45, -36)Placebo once monthly (n = 55) 4 4 4 2 REPATHA 420 mg once monthly (n = 110) -56 -49 -44 -37 Mean difference from placebo
(95% CI)-60
(-68, -52)-53
(-60, -46)-48
(-55, -41)-39
(-45, -33)Figure 5. Effect of REPATHA on LDL-C in Patients with HeFH (Mean % Change from Baseline to Week 12 in RUTHERFORD-2)
N = number of patients randomized and dosed in the full analysis set
Estimates based on a multiple imputation model that accounts for treatment adherence
Error bars indicate 95% confidence intervals
Pediatric Patients with HeFH
Study 6 (HAUSER-RCT, NCT02392559) was a randomized, multicenter, placebo-controlled, double-blind, 24-week trial in 157 pediatric patients aged 10 to 17 years with HeFH [see Use in Specific Populations (8.4)]. HeFH was diagnosed by diagnostic criteria for HeFH [Simon Broome Register Group (1991), the Dutch Lipid Clinic Network (1999), MEDPED (1993)] or by genetic testing. Patients were required to be on a low-fat diet and optimized background lipid-lowering therapy. Patients were randomly assigned 2:1 to receive 24 weeks of subcutaneous once monthly 420 mg REPATHA or placebo; 104 patients received REPATHA and 53 patients received placebo. The mean age was 14 years (range: 10 to 17 years), 56% were female, 85% White, 1% Black, 1% Asian, 13% Other, and 8% Hispanic. The mean LDL-C at baseline was 184 mg/dL; 17% of patients were on high-intensity statin, 62% on moderate-intensity statin, and 13% on ezetimibe.
The difference between REPATHA and placebo in mean percent change in LDL-C from baseline to Week 24 was −38% (95% CI: −45%, −31%; p < 0.0001). For additional results, see Table 8 and Figure 6.
Figure 6. Effect of REPATHA on LDL-C in Pediatric Patients with HeFH (Mean % Change from Baseline in HAUSER-RCT)
EvoMab = evolocumab; LDL-C = low density lipoprotein cholesterol; QM = monthly (subcutaneous)
N = number of patients randomized and dosed in the full analysis set.
Vertical lines represent the standard error around the mean. Plot is based on observed data and no imputation is used for missing values.
Table 8. Effect of REPATHA on Lipid Parameters in Pediatric Patients with HeFH (Mean % Change from Baseline to Week 24 in HAUSER-RCT) Treatment Group LDL-C Non-HDL-C Apo B Total Cholesterol All adjusted p-values < 0.0001.
n = number of patients randomized and dosed in the full analysis set.Placebo once monthly
(n = 53)-6 -6 -2 -5 REPATHA 420 mg once monthly
(n = 104)-44 -41 -35 -32 Mean difference from placebo
(95% CI)-38
(-45, -31)-35
(-42, -28)-32
(-39, -26)-27
(-32, -21)CloseAdults and Pediatric Patients with HoFH
Study 7 (TESLA, NCT01588496) was a multicenter, double-blind, randomized, placebo-controlled, 12-week trial in 49 patients (not on lipid-apheresis therapy) with HoFH. In this trial, 33 patients received subcutaneous injections of 420 mg of REPATHA once monthly and 16 patients received placebo as an adjunct to other lipid-lowering therapies (e.g., statins, ezetimibe). The mean age at baseline was 31 years, 49% were women, 90% White, 4% were Asian, and 6% other. The trial included 10 adolescents (ages 13 to 17 years), 7 of whom received REPATHA. The mean LDL-C at baseline was 349 mg/dL with all patients on statins (atorvastatin or rosuvastatin) and 92% on ezetimibe. The diagnosis of HoFH was made by genetic confirmation or a clinical diagnosis based on a history of an untreated LDL-C concentration > 500 mg/dL together with either xanthoma before 10 years of age or evidence of HeFH in both parents.
The difference between REPATHA and placebo in mean percent change in LDL-C from baseline to Week 12 was −31% (95% CI: −44%, −18%; p < 0.0001). For additional results, see Table 9.
Patients known to have two LDL-receptor negative alleles (little to no residual function) did not respond to REPATHA.
Table 9. Effect of REPATHA on Lipid Parameters in Patients with HoFH (Mean % Change from Baseline to Week 12 in TESLA) Treatment Group LDL-C Non-HDL-C Apo B Total Cholesterol Estimates based on a multiple imputation model that accounts for treatment adherence Placebo once monthly (n = 16) 9 8 4 8 REPATHA 420 mg once monthly (n = 33) -22 -20 -17 -17 Mean difference from placebo
(95% CI)-31
(-44, -18)-28
(-41, -16)-21
(-33, -9)-25
(-36, -14)Study 8 (TAUSSIG, NCT01624142) was a multicenter, open-label 5-year extension study with REPATHA in 106 patients with HoFH, who were treated with REPATHA as an adjunct to other lipid-lowering therapies. The study included 14 pediatric patients (ages 13 to 17 years). All patients in the study were initially treated with REPATHA 420 mg once monthly except for those receiving lipid apheresis at enrollment, who began with REPATHA 420 mg every 2 weeks. Dose frequency in non-apheresis patients could be titrated up to 420 mg once every 2 weeks based on LDL-C response and PCSK9 levels.
A total of 48 patients with HoFH received REPATHA 420 mg once monthly for at least 12 weeks in Study 8 followed by REPATHA 420 mg every 2 weeks for at least 12 weeks. Mean percent change from baseline in LDL-C were −20% at Week 12 of 420 mg once monthly treatment and −30% at Week 12 of 420 mg every 2 weeks treatment, based on available data.
Study 9 (HAUSER-OLE, NCT02624869) was an open-label, single-arm, multicenter, 80-week study to evaluate the safety, tolerability, and efficacy of REPATHA for LDL-C reduction in pediatric patients aged 10 to 17 years with HoFH [see Use in Specific Populations (8.4)]. Patients were on a low-fat diet and receiving background lipid-lowering therapy. Overall, 12 patients with HoFH received 420 mg REPATHA subcutaneously once monthly. The mean age was 12 years (range 11 to 17 years), 17% were female, 75% White, 17% Asian, and 8% Other. Median (Q1, Q3) LDL-C at baseline was 398 (343, 475) mg/dL, and all patients were on statins (atorvastatin or rosuvastatin) and ezetimibe. No patients were receiving lipid apheresis. The diagnosis of HoFH was made by genetic confirmation in all patients but enrollment by a clinical diagnosis was permitted. The median (Q1, Q3) percent change in LDL-C from baseline to Week 80 was −14% (−41, 4). Two of the 3 subjects with < 5% LDLR activity responded to evolocumab treatment.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGREPATHA is a clear to opalescent, colorless to pale yellow solution supplied as follows: Not Made with Natural Rubber Latex – 140 mg/mL prefilled single-dose SureClick® autoinjector2 ...
REPATHA is a clear to opalescent, colorless to pale yellow solution supplied as follows:
Not Made with Natural Rubber Latex – 140 mg/mL prefilled single-dose SureClick® autoinjector 2 pack NDC 72511-393-02 140 mg/mL prefilled single-dose SureClick® autoinjector 1 pack NDC 72511-393-01 140 mg/mL prefilled single-dose Syringe 1 pack NDC 72511-501-01 420 mg/3.5 mL single-dose Pushtronex® system (on-body infusor with prefilled cartridge) 1 pack NDC 72511-770-01 Contains Dry Natural Rubber – - *
- The needle cover of the glass prefilled single-dose SureClick® autoinjector and prefilled single-dose syringe contain dry natural rubber (a derivative of latex) that may cause allergic reactions in individuals sensitive to latex [see Warnings and Precautions (5.1)].
140 mg/mL prefilled single-dose SureClick® autoinjector* 2 pack NDC 72511-760-02 140 mg/mL prefilled single-dose Syringe* 1 pack NDC 72511-750-01 CloseStore refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze. Do not shake.
For convenience, REPATHA may be kept at room temperature at 68°F to 77°F (20°C to 25°C) in the original carton for 30 days. If not used within the 30 days, discard REPATHA.
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient and/or caregiver to read the FDA-Approved Patient Labeling (Patient Information and Instructions for Use). Hypersensitivity - Inform patients that serious hypersensitivity ...
Advise the patient and/or caregiver to read the FDA-Approved Patient Labeling (Patient Information and Instructions for Use).
Hypersensitivity
Inform patients that serious hypersensitivity reactions (e.g., angioedema) have been reported in patients treated with REPATHA. Advise patients on the symptoms of hypersensitivity reactions and instruct them to discontinue REPATHA and seek medical attention promptly, if such symptoms occur.
Latex-Sensitivity
Instruct patients to inform their healthcare provider if they are sensitive to latex. Inform patients that REPATHA is available as prefilled single-dose SureClick® autoinjectors and prefilled single-dose syringes that either contain dry natural rubber (a derivative of latex) in the needle cover or are not made with natural rubber latex, and the carton and Instructions for Use state if the product contains dry natural rubber. Advise latex-sensitive patients that the needle cover of the glass prefilled single-dose SureClick® autoinjector and prefilled single-dose syringe that contain dry natural rubber (a derivative of latex) may cause allergic reactions in individuals sensitive to latex. [see How Supplied/Storage and Handling (16)].
Pregnancy
Advise women who are exposed to REPATHA during pregnancy that there is a pregnancy safety study that monitors pregnancy outcomes. Encourage these patients to report their pregnancy to Amgen at 1-800-77-AMGEN (1-800-772-6436) or https://wwwext.amgen.com/products/global-patient-safety/adverse-event-reporting [see Use in Specific Populations (8.1)].
CloseAdministration
Provide guidance to patients and caregivers on proper subcutaneous administration technique and how to use the prefilled single-dose SureClick® autoinjector, prefilled single-dose syringe, or single-dose on-body infusor with prefilled cartridge correctly. Inform patients that it may take up to 15 seconds to administer REPATHA using the prefilled single-dose SureClick® autoinjector or prefilled single-dose syringe and about 5 minutes to administer REPATHA using the single-dose on-body infusor with prefilled cartridge.
The single-dose on-body infusor with prefilled cartridge is not made with natural rubber latex.
-
SPL UNCLASSIFIED SECTIONFor more information about REPATHA, go to www.REPATHA.com or call 1-844-REPATHA (1-844-737-2842). REPATHA® (evolocumab) Manufactured by: Amgen Inc. One Amgen Center Drive - Thousand Oaks ...
For more information about REPATHA, go to www.REPATHA.com or call 1-844-REPATHA (1-844-737-2842).
REPATHA® (evolocumab)
Manufactured by:
Close
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799
U.S. License Number 1080
Patent: http://pat.amgen.com/repatha/
© 2015-2021, 2024 Amgen Inc. All rights reserved.
v11 -
PATIENT PACKAGE INSERTPatient Information - REPATHA® (ri-PAth-a) (evolocumab) injection, for subcutaneous use - This Patient Information has been approved by the U.S. Food and Drug Administration. v9Revised ...Close
Patient Information
REPATHA® (ri-PAth-a)
(evolocumab)
injection, for subcutaneous useThis Patient Information has been approved by the U.S. Food and Drug Administration.
v9Revised: 11/2024 What is REPATHA? REPATHA is an injectable prescription medicine used: - To reduce the risk of major adverse cardiovascular (CV) events, such as death from cardiovascular disease, heart attack, stroke, certain types of chest pain conditions (unstable angina) requiring hospitalization, or certain types of heart surgery, in adults with cardiovascular disease.
- along with diet alone or together with other cholesterol-lowering medicines in adults with high blood cholesterol levels called primary hyperlipidemia (including a type of high cholesterol called heterozygous familial hypercholesterolemia [HeFH]) to reduce low density lipoprotein (LDL) or bad cholesterol.
- along with diet and other LDL-lowering medicines in children aged 10 years and older with HeFH to reduce LDL cholesterol.
- along with other LDL-lowering medicines in adults and children aged 10 years and older with a type of high cholesterol called homozygous familial hypercholesterolemia (HoFH), to reduce LDL cholesterol.
It is not known if REPATHA is safe and effective in children with HeFH or HoFH who are younger than 10 years of age or in children with other types of hyperlipidemia. Who should not use REPATHA? Do not use REPATHA if you or your child are allergic to evolocumab or to any of the ingredients in REPATHA. See the end of this leaflet for a complete list of ingredients in REPATHA. What should I tell my healthcare provider before using REPATHA? Before you or your child start using REPATHA, tell your healthcare provider about all your medical conditions, including if you or your child: - are allergic to rubber or latex. REPATHA is available as prefilled single-dose SureClick® autoinjectors and prefilled single-dose syringes that either contain dry natural rubber (a derivative of latex) in the needle cover or are not made with natural rubber latex. The carton and "Instructions for Use" will state if your prefilled single-dose SureClick autoinjector or prefilled single-dose syringe contains dry natural rubber.
- The single-dose Pushtronex® system (on-body infusor with prefilled cartridge) is not made with natural rubber latex.
- are pregnant or plan to become pregnant. It is not known if REPATHA will harm your unborn baby. Tell your healthcare provider if you become pregnant while taking REPATHA.
- are breastfeeding or plan to breastfeed. You and your healthcare provider should decide if you will take REPATHA or breastfeed.
If you or your child are pregnant or breastfeed during REPATHA treatment, you are encouraged to call Amgen at 1-800-772-6436 (1-800-77-AMGEN) or visit https://wwwext.amgen.com/products/global-patient-safety/adverse-event-reporting to share information about the health of you and your baby or your child and your child's baby. Tell your healthcare provider or pharmacist about any prescription and over-the-counter medicines, vitamins, or herbal supplements you or your child take. How should I use REPATHA? - See the detailed "Instructions for Use" that comes with this Patient Information about the right way to prepare and give REPATHA.
- Use REPATHA exactly as your healthcare provider tells you or your child to use it.
- REPATHA is given under the skin (subcutaneously), every 2 weeks or 1 time each month.
- If you or your child have HoFH, the recommended starting dose is 420 mg one time each month. After 12 weeks, your healthcare provider may decide to increase the dose to 420 mg every two weeks. If you or your child receive lipid apheresis, your healthcare provider may decide to start you or your child on a dose of 420 mg every two weeks to match with the apheresis treatment and you or your child should take the dose after the apheresis treatment.
- REPATHA comes as a prefilled single-dose (1 time) autoinjector (SureClick autoinjector), as a prefilled single-dose syringe or as a single-dose Pushtronex system (on-body infusor with prefilled cartridge). Your healthcare provider will prescribe the type and dose that is best for you or your child.
- If your healthcare provider prescribes you or your child the 420 mg dose, you or your child may use:
- a single-dose on-body infusor with prefilled cartridge to give the injection over 5 minutes, or
- 3 separate injections in a row, using a different prefilled single-dose SureClick autoinjector or prefilled single-dose syringe for each injection. Give all of these injections within 30 minutes.
- If your healthcare provider decides that you or your child or a caregiver can give REPATHA, you or your child or your caregiver should receive training on the right way to prepare and inject REPATHA. Do not try to inject REPATHA until you or your child have been shown the right way by your healthcare provider or nurse.
- If you or your child are using the prefilled single-dose SureClick autoinjector, put the yellow safety guard (needle inside) of the prefilled single-dose SureClick autoinjector on the skin before injecting.
- You or your child can inject into the thigh, upper arm, or stomach (abdomen), except for a two-inch area around the belly button.
- Do not choose an area where the skin is tender, bruised, red, or hard. Avoid injecting into areas with scars or stretch marks.
- Always check the label of your prefilled single-dose SureClick autoinjector, prefilled single-dose syringe, or single-dose on-body infusor with prefilled cartridge to make sure you have the correct medicine and the correct dose of REPATHA before each injection.
- If you or your child forget to use REPATHA or are not able to take the dose at the regular time, inject your or your child's missed dose as soon as you remember, as long as it is within 7 days of the missed dose.
- If it is more than 7 days from the missed dose and you or your child are using the every-2-week dose, inject the next dose based on the original schedule. This will put you or your child back on the original schedule.
- If it is more than 7 days from the missed dose and you or your child are using the 1 time each-month dose, inject the dose and start a new schedule using this date.
If you or your child are not sure when to take REPATHA after a missed dose, ask your healthcare provider or pharmacist. - If your healthcare provider has prescribed REPATHA along with other cholesterol-lowering medicines for you or your child, follow instructions from your healthcare provider. Read the Patient Information for those medicines.
- If you or your child use more REPATHA than you should, talk to your healthcare provider or pharmacist.
- Do not stop using REPATHA without talking with your healthcare provider. If you or your child stop using REPATHA, the cholesterol levels can increase.
What are the possible side effects of REPATHA? REPATHA can cause serious side effects including: -
Serious Allergic Reactions. Some people taking REPATHA have had serious allergic reactions. Stop taking REPATHA and call your healthcare provider or seek emergency medical help right away if you or your child have any of these symptoms:
- trouble breathing or swallowing
- raised bumps (hives)
- rash, or itching
- swelling of the face, lips, tongue, throat or arms
The most common side effects of REPATHA include: runny nose, sore throat, symptoms of the common cold, flu or flu-like symptoms, back pain, high blood sugar levels (diabetes) and redness, pain, or bruising at the injection site. Tell your healthcare provider if you or your child have any side effect that bothers you or that does not go away. These are not all the possible side effects of REPATHA. Ask your healthcare provider or pharmacist for more information. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store REPATHA? - Store REPATHA in the refrigerator between 36°F to 46°F (2°C to 8°C). Store REPATHA in the original carton until use to protect it from light.
- If needed, REPATHA can be stored at room temperature between 68°F to 77°F (20°C to 25°C) in the original carton for up to 30 days. Throw away REPATHA that has been stored at room temperature for more than 30 days.
- Do not freeze REPATHA.
- Do not shake REPATHA.
General information about the safe and effective use of REPATHA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use REPATHA for a condition for which it was not prescribed. Do not give REPATHA to other people, even if they have the same symptoms that you or your child have. It may harm them. You can ask your pharmacist or healthcare provider for information about REPATHA that is written for healthcare professionals. What are the ingredients in REPATHA? - Active Ingredient: evolocumab
- Inactive Ingredients: proline; acetate; polysorbate 80; water for injection, USP; and sodium hydroxide.
Manufactured by: Amgen Inc. One Amgen Center Drive, Thousand Oaks, California 91320-1799. U.S. License Number 1080 Patent: http://pat.amgen.com/repatha/ © 2017-2021, 2024 Amgen Inc. All rights reserved. For more information about REPATHA, go to www.REPATHA.com or call 1-844-REPATHA (1-844-737-2842). -
INSTRUCTIONS FOR USEInstructions for Use: Pushtronex® System for - Repatha® (ri-PAth-a) (evolocumab) Single-Dose On-Body Infusor and Prefilled Cartridge - Guide to parts - Prefilled Cartridge - On-Body ...
Instructions for Use:
Pushtronex® System for
Repatha® (ri-PAth-a)
(evolocumab)
Single-Dose On-Body Infusor and Prefilled CartridgeGuide to parts Prefilled Cartridge 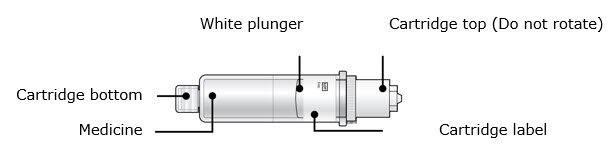
On-Body Infusor Front view 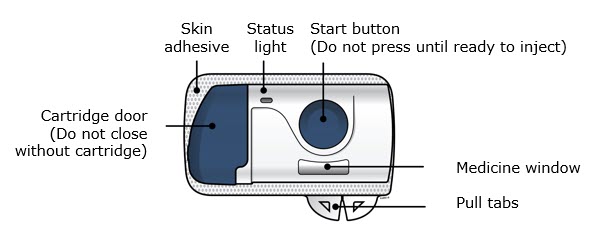
Back view 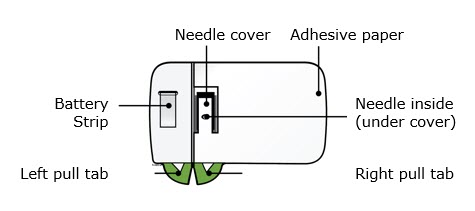
Important: Needle is inside. Important Before you use the on-body infusor and prefilled cartridge for use with Repatha (evolocumab), read this important information: - It is important that you do not try to give yourself the injection unless you have received training from your healthcare provider.
- Children who are 10 to 17 years of age should use the on-body infusor and prefilled cartridge under adult supervision, as instructed by the healthcare provider.
Storing your on-body infusor and prefilled cartridge - Keep the on-body infusor and prefilled cartridge in the original carton to protect from light or physical damage.
- The on-body infusor and prefilled cartridge must be kept in the refrigerator 36°F to 46°F (2°C to 8°C).
- For your injection, take your on-body infusor and prefilled cartridge out of the refrigerator and let them sit at room temperature for at least 45 minutes before you inject.
- After you remove the on-body infusor and prefilled cartridge from the refrigerator, they should be kept at room temperature at 68°F to 77°F (20°C to 25°C) in the original carton and must be used within 30 days.
- Do not store the on-body infusor and prefilled cartridge in temperatures above 77°F (25°C) such as in your vehicle's glove box or trunk. Do not freeze.
Using your on-body infusor and prefilled cartridge - Do not shake the on-body infusor or prefilled cartridge.
- Do not remove the on-body infusor and prefilled cartridge from the box or clear tray until you are ready to inject.
- Do not touch the start button until you place the loaded on-body infusor and prefilled cartridge onto your skin and are ready to inject.
- After you insert the cartridge into the on-body infusor, make sure you give your injection within 5 minutes. Waiting longer than 5 minutes can dry out the medicine.
- You can only press the start button 1 time. If an error occurs, the on-body infusor cannot be used.
- Do not use the on-body infusor and prefilled cartridge if either has been dropped onto a hard surface. Part of the on-body infusor and prefilled cartridge may be broken even if you cannot see the break. Use a new on-body infusor and prefilled cartridge.
- Do not reuse the on-body infusor and prefilled cartridge. The on-body infusor and prefilled cartridge are for single-dose only.
- Do not let the on-body infusor get wet from water or any other liquids. It contains electronics that should not get wet.
- The single-dose on-body infusor for subcutaneous injection is made to only be used with the prefilled cartridge.
- Moderate physical activities can be done during the injection process, such as walking, reaching and bending.
- Do not use the on-body infusor and prefilled cartridge after the expiration date on the carton.
- The on-body infusor and prefilled cartridge are not made with natural rubber latex.
A healthcare provider who knows how to use the on-body infusor should be able to answer your questions. For more information, call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com.
Keep the on-body infusor and prefilled cartridge out of the reach of children.Step 1: Prepare 1A Remove the on-body infusor and prefilled cartridge carton from the refrigerator. Wait at least 45 minutes before injecting for the on-body infusor and prefilled cartridge in the carton to naturally reach room temperature. - Do not try to warm the prefilled cartridge by using a heat source such as hot water or a microwave.
In any above cases, use a new on-body infusor and prefilled cartridge and call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com. 1B Open the carton and peel away the white paper cover. Remove the plastic cover from the clear tray. 
Leave the on-body infusor and prefilled cartridge in the clear tray until you are ready to inject. - Do not touch the start button until the on-body infusor is on the skin and you are ready to inject.
- Do not use if the white paper cover is missing or damaged.
1C Gather all materials needed for your injection and then wash your hands well with soap and water. On a clean, well-lit work surface, place the: - Clear tray containing the on-body infusor and prefilled cartridge
- Alcohol wipes
- Cotton ball or gauze pad
- Adhesive bandage
- Sharps disposal container

1D To securely attach the on-body infusor, prepare and clean an injection site that is less likely to have body hair, or you can trim the area. Use a firm and flat skin surface. You can use: - Your thigh
- Stomach area (abdomen), except for a two-inch area right around your navel
- Outer area of upper arm (only if someone else is giving the injection)

Clean your injection site with an alcohol wipe. Let your skin dry. - Do not touch this area again before injecting.
- Do not inject into areas where the skin is tender, bruised, red or hard. Avoid injecting into areas with wrinkles, skin folds, scars, stretch marks, moles and excessive hair.
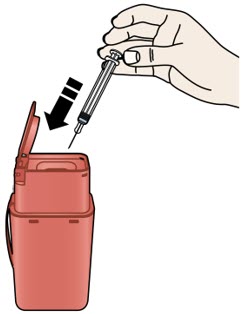
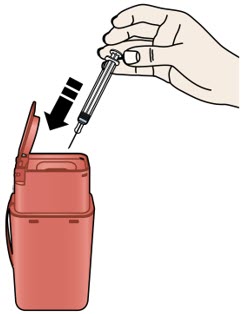
Important: To attach the on-body infusor securely, it is important to use a firm and flat skin surface. Step 2: Get ready 2A Open the on-body infusor by swinging the cartridge door to the right. Then, leave the door open. Do not close the cartridge door before the cartridge is loaded. If you accidently close the cartridge door, press on the left side of the door to release the door latch.
If you are still unable to open the door, call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com.
Do not press the start button until you are ready to inject.
2B Inspect the cartridge. 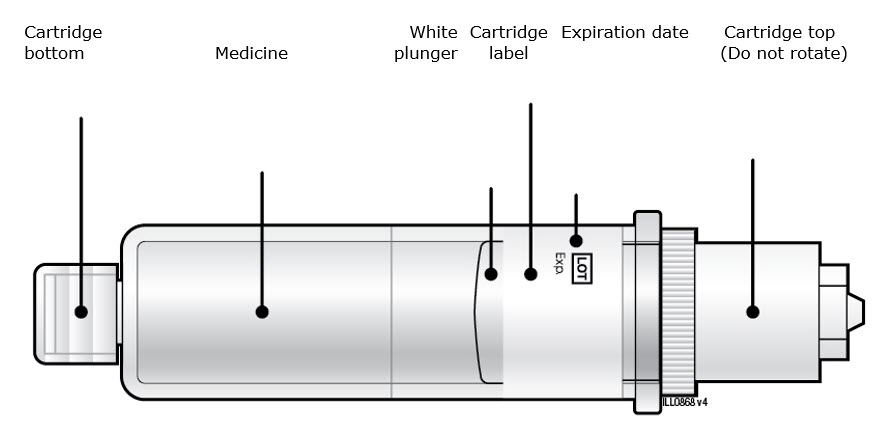
Check the expiration date: do not use if this date has passed.
Make sure the medicine in the cartridge is clear and colorless to slightly yellow.- Do not use if the medicine is cloudy or discolored or contains flakes or particles.
- Do not use if any part of the cartridge looks cracked or broken.
- Do not use if pieces of the cartridge are missing or not securely attached.
In any above cases, use a new on-body infusor and prefilled cartridge and call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com. 2C Clean the cartridge bottom.  Grab Here
Grab HereWith 1 hand, hold the cartridge barrel and clean the cartridge bottom with an alcohol wipe. - Do not remove or rotate the cartridge top or bottom.
- Do not touch the bottom of the cartridge after cleaning with alcohol wipe.
2D Load the cleaned cartridge into the on-body infusor and firmly press on the top until it is secured in place. Make sure that you give your injection within 5 minutes after loading the cartridge. Do not insert the cartridge more than 5 minutes before injection. This can dry out the medicine. 
Insert the cartridge bottom first. - Do not touch the start button until you have placed the loaded on-body infusor on your skin.
2E Swing the door to the left. Then, squeeze firmly until it snaps shut. Apply enough pressure when closing the door and make sure there is a "snap" before going to the next step. 
Make sure the cartridge fits securely in the on-body infusor before you close the door. - Do not close the door if the cartridge is missing or not fully inserted.
- Do not touch the start button until you have placed the loaded on-body infusor on your skin.
Step 3: Inject 3A Peel away both green pull tabs to show the adhesive. The on-body infusor is on when the blue status light flashes. 
You must remove both green pull tabs to turn the loaded on-body infusor on. You will hear beeping and see a flashing blue status light. -
Do not pull the skin adhesive backing off the on-body infusor.
-
Do not touch the skin adhesive.
-
Do not touch the start button until you have placed the loaded on-body infusor on your skin.
-
Do not touch the needle cover area.
-
Do not place the loaded on-body infusor on your body if the red status light flashes continuously.
- Do not fold the skin adhesive over onto itself.
3B Choose your on-body infusor injection site. Only use the outer arm if someone else is giving the injection. Stomach area placement Thigh placement 
or 
Stretch method for stomach Do not stretch for thigh Important: Adjust your body posture to avoid skin folds and bulges. 3C When the blue light flashes, the on-body infusor is ready. Keep the stretch (stomach area method only). Hold the loaded on-body infusor with the blue light visible, and place it on your skin. You may hear beeps. Stomach area placement Thigh placement 
or 
The loaded on-body infusor will lay flat on your body. Make sure all of the adhesive is attached to your skin. Run a finger around the adhesive edges to secure it. Make sure clothing does not get in the way of the loaded on-body infusor, and you can see the blue light at all times. - Do not move the loaded on-body infusor after it has been placed onto your skin.
3D Firmly press and release the start button. A flashing green light and a click signals the injection has started. 
- You may hear a pumping sound.
- You may feel a pinch.
- Make sure you see a green, flashing status light.
- You may hear beeps that mean your injection has started.
3E The injection takes about 5 minutes to finish. The status light turns solid green, and the device beeps, when done. 
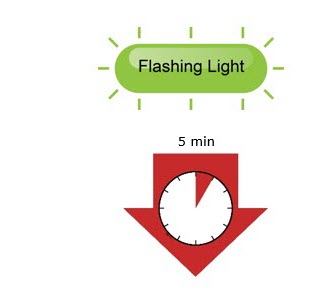
It is okay to hear a pumping sound start and stop during injection. 
Injection is finished when: - The status light changes to solid green.
- You hear several beeps.
- The plunger fills medicine window all the way.

Step 4: Finish 4A When the injection is done, grab the skin adhesive to carefully peel the on-body infusor off skin. After removal, check the medicine window. The green light should now be off. Used plunger filling medicine window 
Check to see that the used plunger fills the medicine window all the way, and the green solid light turns off, letting you know all medicine has been injected. If the plunger did not fill the window, call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com. - The used on-body infusor will beep when removed from your skin.
- It is normal to see a few drops of fluid on your skin after you remove the used on-body infusor.
4B Throw away the used on-body infusor in a sharps container. - The on-body infusor contains batteries, electronics, and a needle.
- Put the used on-body infusor in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the on-body infusor in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Do not recycle the on-body infusor or sharps disposal container or throw them into household trash.

4C Check the injection site. If there is blood, press a cotton ball or gauze pad on your injection site. Do not rub the injection site. Apply an adhesive bandage if needed. Troubleshooting What do I do if the loaded on-body infusor status light continuously flashes red and I hear beeps? 
Stop using the loaded on-body infusor. If the on-body infusor is attached to your body, carefully remove it. Call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com. This Instructions for Use has been approved by the U.S. Food and Drug Administration. Commonly Asked Questions What if I hear the on-body infusor beep and see a red blinking light when it is on my body? This means that an error has happened. When this happens, the injection will stop by itself. Remove the on-body infusor from your body by slowly and carefully peeling it off of your skin, call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com. What should I do if the on-body infusor comes off my body during the injection? Though unlikely, if the on-body infusor comes off during the injection, the on-body infusor will make a beeping sound, you will see the blinking red light, and the on-body infusor will stop. The loaded on-body infusor can no longer be used, and do not reapply to your body. Call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com. What if I push the start button before I place the on-body infusor on my skin? If you have removed the adhesive backing and pressed the start button, the on-body infusor will make a beeping sound and you will see the blinking red light. The on-body infusor will stop. Stop using the on-body infusor, call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com. What if the on-body infusor does not beep and the blue status light does not blink when I remove the pull tabs? Check to see if both green pull tabs have been fully removed from the on-body infusor, including the adhesive paper over the battery strip and needle cover. If both green pull tabs have been fully removed and the on-body infusor still does not turn on, use a new on-body infusor and prefilled cartridge. Call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com. What if I push the start button and nothing happens? Remove the on-body infusor by slowly and carefully peeling it away from your skin. Do not reapply the same on-body infusor that you have already placed on your skin. Call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com. What if I cannot open the cartridge door to insert the cartridge? To open the on-body infusor door, press on the left side of the door to release the door latch. If you are still unable to open the door, call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com. Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, CA 91320-1799
© 2015-2021 Amgen Inc.
All rights reserved.
<part number> Revised: 9/2021 v8U.S. License Number 1080
Additional environmental conditions
Relative humidity range is 15% to 85%.
Altitude range is -984 feet to 11483 feet (-300 meters to 3500 meters).
During injection, keep the on-body infusor a minimum of 12 inches (30 cm) away from other electronics such as cellular phones.
Warning: Do not modify the device.
Warning: Do not expose the on-body infusor for Repatha to Magnetic Resonance (MR) Environment (e.g., MRI).
On-body infusor operating temperature range is 59°F to 104°F (15°C to 40°C).
On-body infusor complies with IEC 60601-1 3.1 edition and IEC 60601-1-2: 2014.
www.devicepatents.com.CloseSYMBOL TABLE 




Do not re-use Serial number Type BF Applied Part Do not use if packaging is damaged On-Body Infusor containing 420 mg/3.5 mL (120 mg/mL) 




Sterilized using ethylene oxide Refer to Instructions for Use Lot number Keep dry Open here 
MR Unsafe -
INSTRUCTIONS FOR USE REPATHA® (ri-PAth-a) (evolocumab) injection, for subcutaneous use 140 mg/mL single-dose prefilled SureClick® autoinjector(contains dry natural rubber)This Instructions for Use contains information on how to inject REPATHA with a SureClick autoinjector. If your healthcare provider decides that you or a caregiver may be able to give your ...
This Instructions for Use contains information on how to inject REPATHA with a SureClick autoinjector.
If your healthcare provider decides that you or a caregiver may be able to give your injections of REPATHA at home, you should receive training on the right way to prepare and inject REPATHA. Do not try to inject yourself until you have been shown the right way to give the injections by your healthcare provider or nurse.
The medicine in the REPATHA autoinjector is for injection under the skin (subcutaneous injection). See the REPATHA Patient Information for information about REPATHA.
Getting to know your prefilled autoinjector 
1. Important Information You Need to Know Before Injecting REPATHA - It is important that you do not try to give the injection until you have fully read and understood this Instructions for Use.
- The orange cap on the REPATHA SureClick autoinjector contains a needle cover (located inside the cap) that contains dry natural rubber, which is made from latex. Tell your healthcare provider if you are allergic to latex.
- Do not use the autoinjector if the carton is damaged or the seal is broken.
- Do not use the autoinjector after the expiration date on the label.
- Do not shake the autoinjector.
- Do not remove the orange cap from the autoinjector until you are ready to inject.
- Do not use the autoinjector if it has been frozen.
- Do not use the autoinjector if it has been dropped on a hard surface. Part of the autoinjector may be broken even if you cannot see the break. Use a new autoinjector and call 1-844-REPATHA (1-844-737-2842).
Frequently asked questions:
For additional information and answers to frequently asked questions, visit www.repatha.com.Where to get help:
If you want more information or help using REPATHA:- Contact your healthcare provider,
- Visit www.repatha.com, or
- Call 1-844-REPATHA (1-844-737-2842)
2. Storing and Preparing to Inject REPATHA 
2a Refrigerate the autoinjector carton until you are ready to use it. - Keep the autoinjector in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the autoinjector in the original carton to protect it from light or physical damage.
- Do not freeze the autoinjector.
- Do not store the autoinjector in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.
Important: Keep the autoinjector and all medicines out of the sight and reach of children. WAIT 
2b Wait 30 minutes for the autoinjector to reach room temperature. - Remove the number of autoinjectors you need for your injection and put any unused autoinjectors back into the refrigerator.
- Let the autoinjector warm up naturally.
- Do not heat the autoinjector with hot water, a microwave, or direct sunlight.
- Do not shake the autoinjector at any time.
- Using the autoinjector at room temperature makes sure the full dose is delivered and allows for a more comfortable injection.

2c You may keep REPATHA at room temperature for up to 30 days, if needed. - For example, when you are traveling, you may keep REPATHA at room temperature.
- Keep it at room temperature between 68°F to 77°F (20°C to 25°C) in the original carton.
- Record the date you removed it from the refrigerator and use it within 30 days.
Important: Place the autoinjector in a sharps disposal container if it has reached room temperature and has not been used within 30 days. 
2d Inspect the medicine. It should be clear and colorless to slightly yellow. - It is okay to see air bubbles in the autoinjector.
- Do not use REPATHA if the medicine is cloudy, discolored, or has flakes or particles.
Important: If the medicine is cloudy, discolored, or has flakes or particles, or if the autoinjector is damaged or expired, call 1-844-REPATHA (1-844-737-2842). 
2e Check the expiration date (Exp.) and inspect the autoinjector for damage. - Do not use the autoinjector if the expiration date has passed.
-
Do not use the autoinjector if:
- the orange cap is missing or loose.
- it has cracks or broken parts.
- it has been dropped on a hard surface.
- Make sure you have the right medicine and dose.
3. Getting Ready for Your Injection 
3a Gather and place the following items for your injection on a clean, flat, and well-lit surface: - REPATHA autoinjector (room temperature)
- Sharps disposal container [see Disposing of REPATHA and Checking the Injection Site]
- Alcohol wipe
- Adhesive bandage
- Cotton ball or gauze pad

3b Inject into 1 of these sites. - Inject into the front of your thigh or stomach (except for 2 inches around your belly button).
- Someone else can inject in your thigh, stomach, or back of your upper arm.
- Choose a different site for each injection.
Important: Avoid areas with scars or stretch marks, or where the skin is tender, bruised, red, hard, raised, thick or scaly skin patch or lesion. 
3c Wash hands thoroughly with soap and water. 
3d Clean the injection site with an alcohol wipe. - Let the skin dry on its own.
- Do not touch this area again before injecting.
4. Injecting REPATHA Important: Only remove the orange cap when you can inject right away (within 5 minutes) because the medicine can dry out. Do not recap. 
4a Grasp the autoinjector so you can see the window. Pull the orange cap straight off. You may need to pull hard. - Do not twist, bend or wiggle the orange cap to pull it off.
- Never put the orange cap back on. It may damage the needle.
- Do not put your finger inside the yellow safety guard.
- It is normal to see a drop of medicine at the end of the needle or yellow safety guard.

4b Stretch or pinch the skin to create a firm surface at the injection site. Place the yellow safety guard straight against the skin. - Keep the skin stretched or pinched until the injection is finished.
- Make sure you can see the window.
- Make sure the autoinjector is positioned straight on the injection site (at a 90-degree angle).
PUSH
and hold against skin
4c Firmly push the autoinjector down until the yellow safety guard stops moving. Hold the autoinjector down, do not lift. - The yellow safety guard pushes in and unlocks the gray start button.
PRESS
gray start button
4d Keep pushing the autoinjector down and press the gray start button to start the injection. - You may hear or feel a click.
- The window starts to turn yellow.
- It is okay to let go of the gray start button.
WATCH and CONFIRM
that the window turns fully yellow
4e Keep pushing the autoinjector down. When the window is fully yellow, the injection is complete. - The injection may take up to 15 seconds to complete.
- You may hear or feel a click.
- Lift the autoinjector away from your skin.
- The yellow safety guard locks around the needle.
Important: If the window has not turned fully yellow, or it looks like the medicine is still coming out, you have not received a full dose. Call your healthcare provider right away. 5a Place the used autoinjector and orange cap in an FDA-cleared sharps disposal container right away after use. Important: Do not throw away the autoinjector in your household trash. - Do not reuse the autoinjector.
- Do not touch the yellow safety guard.

5b Check the injection site. - Do not rub the injection site.
- If there is blood, press a cotton ball or gauze pad on your injection site. Apply an adhesive bandage if necessary.
Additional information about your sharps disposal container
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
Disposing of sharps disposal containers:
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you
live in, go to the FDA's website at:
http://www.fda.gov/safesharpsdisposalDo not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
Important: Keep the autoinjector and sharps disposal container out of the sight and reach of children.
For more information or help call 1-844-REPATHA (1-844-737-2842).
REPATHA (evolocumab)
AMGEN®
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799
U.S. License Number 1080
© 2015-2022, 2024 Amgen Inc. All rights reserved.
<Part number>This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Close
Revised: 11/2024
v11 -
INSTRUCTIONS FOR USE REPATHA® (ri-PAth-a) (evolocumab) injection, for subcutaneous use 140 mg/mL single-dose prefilled SureClick® autoinjectorThis Instructions for Use contains information on how to inject REPATHA with a SureClick autoinjector. If your healthcare provider decides that you or a caregiver may be able to give your ...
This Instructions for Use contains information on how to inject REPATHA with a SureClick autoinjector.
If your healthcare provider decides that you or a caregiver may be able to give your injections of REPATHA at home, you should receive training on the right way to prepare and inject REPATHA. Do not try to inject yourself until you have been shown the right way to give the injections by your healthcare provider or nurse.
The medicine in the REPATHA autoinjector is for injection under the skin (subcutaneous injection). See the REPATHA Patient Information for information about REPATHA.
Getting to know your prefilled autoinjector 
1. Important Information You Need to Know Before Injecting REPATHA - It is important that you do not try to give the injection until you have fully read and understood this Instructions for Use.
- Do not use the autoinjector if the carton is damaged or the seal is broken.
- Do not use the autoinjector after the expiration date on the label.
- Do not shake the autoinjector.
- Do not remove the orange cap from the autoinjector until you are ready to inject.
- Do not use the autoinjector if it has been frozen.
- Do not use the autoinjector if it has been dropped on a hard surface. Part of the autoinjector may be broken even if you cannot see the break. Use a new autoinjector and call 1-844-REPATHA (1-844-737-2842).
- The autoinjector is not made with natural rubber latex.
Frequently asked questions:
For additional information and answers to frequently asked questions, visit www.repatha.com.Where to get help:
If you want more information or help using REPATHA:- Contact your healthcare provider,
- Visit www.repatha.com, or
- Call 1-844-REPATHA (1-844-737-2842)
2. Storing and Preparing to Inject REPATHA 
2a Refrigerate the autoinjector carton until you are ready to use it. - Keep the autoinjector in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the autoinjector in the original carton to protect it from light or physical damage.
- Do not freeze the autoinjector.
- Do not store the autoinjector in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.
Important: Keep the autoinjector and all medicines out of the sight and reach of children. WAIT 
2b Wait 30 minutes for the autoinjector to reach room temperature. - Remove the number of autoinjectors you need for your injection and put any unused autoinjectors back into the refrigerator.
- Let the autoinjector warm up naturally.
- Do not heat the autoinjector with hot water, a microwave, or direct sunlight.
- Do not shake the autoinjector at any time.
- Using the autoinjector at room temperature makes sure the full dose is delivered and allows for a more comfortable injection.

2c You may keep REPATHA at room temperature for up to 30 days, if needed. - For example, when you are traveling, you may keep REPATHA at room temperature.
- Keep it at room temperature between 68°F to 77°F (20°C to 25°C) in the original carton.
- Record the date you removed it from the refrigerator and use it within 30 days.
Important: Place the autoinjector in a sharps disposal container if it has reached room temperature and has not been used within 30 days. 
2d Inspect the medicine. It should be clear and colorless to slightly yellow. - It is okay to see air bubbles in the autoinjector.
- Do not use REPATHA if the medicine is cloudy, discolored, or has flakes or particles.
Important: If the medicine is cloudy, discolored, or has flakes or particles, or if the autoinjector is damaged or expired, call 1-844-REPATHA (1-844-737-2842). 
2e Check the expiration date (Exp.) and inspect the autoinjector for damage. - Do not use the autoinjector if the expiration date has passed.
-
Do not use the autoinjector if:
- the orange cap is missing or loose.
- it has cracks or broken parts.
- it has been dropped on a hard surface.
- Make sure you have the right medicine and dose.
3. Getting Ready for Your Injection 
3a Gather and place the following items for your injection on a clean, flat, and well-lit surface: - REPATHA autoinjector (room temperature)
- Sharps disposal container [see Disposing of REPATHA and Checking the Injection Site]
- Alcohol wipe
- Adhesive bandage
- Cotton ball or gauze pad

3b Inject into 1 of these sites. - Inject into the front of your thigh or stomach (except for 2 inches around your belly button).
- Someone else can inject in your thigh, stomach, or back of your upper arm.
- Choose a different site for each injection.
Important: Avoid areas with scars or stretch marks, or where the skin is tender, bruised, red, hard, raised, thick or scaly skin patch or lesion. 
3c Wash hands thoroughly with soap and water. 
3d Clean the injection site with an alcohol wipe. - Let the skin dry on its own.
- Do not touch this area again before injecting.
4. Injecting REPATHA Important: Only remove the orange cap when you can inject right away (within 5 minutes) because the medicine can dry out. Do not recap. 
4a Grasp the autoinjector so you can see the window. Pull the orange cap straight off. You may need to pull hard. - Do not twist, bend or wiggle the orange cap to pull it off.
- Never put the orange cap back on. It may damage the needle.
- Do not put your finger inside the yellow safety guard.
- It is normal to see a drop of medicine at the end of the needle or yellow safety guard.

4b Stretch or pinch the skin to create a firm surface at the injection site. Place the yellow safety guard straight against the skin. - Keep the skin stretched or pinched until the injection is finished.
- Make sure you can see the window.
- Make sure the autoinjector is positioned straight on the injection site (at a 90-degree angle).
PUSH
and hold against skin
4c Firmly push the autoinjector down until the yellow safety guard stops moving. Hold the autoinjector down, do not lift. - The yellow safety guard pushes in and unlocks the gray start button.
PRESS
gray start button
4d Keep pushing the autoinjector down and press the gray start button to start the injection. - You may hear or feel a click.
- The window starts to turn yellow.
- It is okay to let go of the gray start button.
WATCH and CONFIRM
that the window turns fully yellow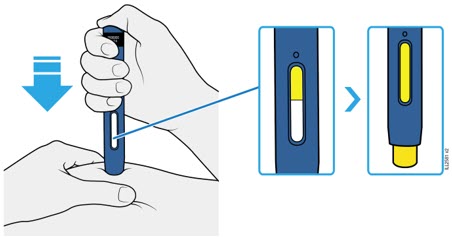
4e Keep pushing the autoinjector down. When the window is fully yellow, the injection is complete. - The injection may take up to 15 seconds to complete.
- You may hear or feel a click.
- Lift the autoinjector away from your skin.
- The yellow safety guard locks around the needle.
Important: If the window has not turned fully yellow, or it looks like the medicine is still coming out, you have not received a full dose. Call your healthcare provider right away. 5a Place the used autoinjector and orange cap in an FDA-cleared sharps disposal container right away after use. Important: Do not throw away the autoinjector in your household trash. - Do not reuse the autoinjector.
- Do not touch the yellow safety guard.
5b Check the injection site. - Do not rub the injection site.
- If there is blood, press a cotton ball or gauze pad on your injection site. Apply an adhesive bandage if necessary.
Additional information about your sharps disposal container
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
Disposing of sharps disposal containers:
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you
live in, go to the FDA's website at:
http://www.fda.gov/safesharpsdisposalDo not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
Important: Keep the autoinjector and sharps disposal container out of the sight and reach of children.
For more information or help call 1-844-REPATHA (1-844-737-2842).
REPATHA (evolocumab)
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799
U.S. License Number 1080
© 2024 Amgen Inc. All rights reserved.
<Part number>This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Close
Issued: 11/2024
v1 -
REFERENCE GUIDESide 1Reference Guide - Repatha® (ri-PAth-a) (evolocumab) injection, for subcutaneous use - Single-Dose Prefilled SureClick® Autoinjector - 140 mg/mLRead all instructions in carton before ...
Side 1 Reference Guide
Repatha® (ri-PAth-a) (evolocumab)
injection, for subcutaneous use
Single-Dose Prefilled SureClick® Autoinjector
140 mg/mLRead all instructions in carton before use.
Questions? Call 1-844-REPATHA
(1-844-737-2842)Guide to parts Wait at least 30 minutes for the autoinjector to come to room temperature before giving your injection. Pull the orange cap straight off only when you are ready to inject.
Do not leave the orange cap off for more than 5 minutes. This can dry out the medicine.
2 
Prepare and clean your injection site. 1 
Turn over to continue CloseSide 2 Reference Guide
Repatha® (ri-PAth-a) (evolocumab)
injection, for subcutaneous use
Single-Dose Prefilled SureClick® Autoinjector
140 mg/mLAMGEN® Read other side first Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, CA 91320-1799
© 2019-2022 Amgen Inc. All rights reserved.
 This printed material is recyclable.
This printed material is recyclable.
<part number> Revised: 8/2022 v4Stretch or pinch your injection site to create a firm surface. Put the yellow safety guard (needle inside) on your skin at 90 degrees. Firmly push down the autoinjector onto your skin until it stops moving. Keep pushing down on your skin for about 15 seconds. 3 4 5 6 

When you are ready to inject, press the gray start button. You will hear a click.


Window turns from clear to yellow when the injection is done.

-
INSTRUCTIONS FOR USEInstructions for Use - Repatha® (ri-PAth-a) (evolocumab) prefilled single-dose syringe - (contains dry natural rubber) Getting to know the prefilled syringe - Before useAfter ...
Instructions for Use
Repatha® (ri-PAth-a)
(evolocumab)
prefilled single-dose syringe
(contains dry natural rubber)Getting to know the prefilled syringe
Before use After use 

Needle is inside Important Before you use a prefilled single-dose syringe, read this important information: - It is important that you do not try to give the injection until you have fully read and understood this Instructions for Use.
- Check the carton, prefilled syringe label, and prescription to make sure you have the correct medicine and dose.
- Check the expiration date on the REPATHA prefilled syringe carton: do not use if this date has passed.
- It is important that you do not try to give yourself or someone else the injection unless you have received training from your healthcare provider.
- The gray needle cap on the prefilled syringe contains dry natural rubber, which is made from latex. Tell your healthcare provider if you are allergic to latex.
- Keep the prefilled syringe in the original carton to protect from light during storage.
- Keep the prefilled syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- If removed from the refrigerator, the prefilled syringe should be kept at room temperature at 68°F to 77°F (20°C to 25°C) in the original carton and must be used within 30 days. Place the prefilled syringe in a sharps disposal container if it has reached room temperature and has not been used within 30 days.
- Do not freeze the prefilled syringe or use a prefilled syringe that has been frozen.
- Do not store the prefilled syringe in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.
Do not: - Do not use the prefilled syringe if the packaging is open or damaged.
- Do not remove the gray needle cap from the prefilled syringe until you are ready to inject.
- Do not use the prefilled syringe if it has been dropped onto a hard surface. Part of the prefilled syringe may be broken even if you cannot see the break. Use a new prefilled syringe and call 1-844-REPATHA (1-844-737-2842).
- Do not use the prefilled syringe after the expiration date.
A healthcare provider who knows how to use the prefilled syringe should be able to answer your questions. For more information, call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com.
Keep the prefilled syringe and all medicines out of the sight and reach of children.Step 1: Prepare 1 A Remove the prefilled syringe carton from the refrigerator and wait 30 minutes. Wait at least 30 minutes for the prefilled syringe in the carton to reach room temperature before injecting. 
Using the prefilled syringe at room temperature makes sure the full dose is delivered and allows for a more comfortable injection. Do not heat the syringe. Let it warm up on its own naturally. Do not try to warm the prefilled syringe by using a heat source such as hot water or microwave. Do not leave the prefilled syringe in direct sunlight. Do not shake the prefilled syringe. 1 B Gather all materials needed for your injection. Wash your hands thoroughly with soap and water. On a clean, well-lit, flat work surface, place: - 1 REPATHA prefilled syringe in carton
- Alcohol wipes
- Cotton ball or gauze pad
- Adhesive bandage
- Sharps disposal container (see Step 4: Finish)
1 C Choose your injection site. 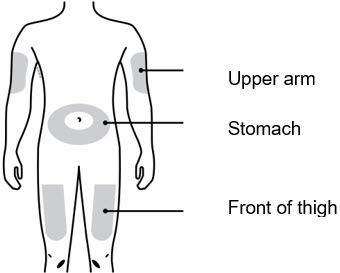
You can use the: - front of your thigh
- stomach (abdomen), except for a 2 inch area around your belly button
Do not choose an area where the skin is tender, bruised, red, or hard. Avoid injecting into areas with scars or stretch marks.
Choose a different site each time you give yourself an injection.1 D Clean your injection site. 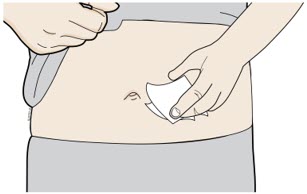
Clean your injection site with an alcohol wipe. Let your skin dry before injecting.
Do not touch this area of skin again before injecting.1 E Remove prefilled syringe from tray. 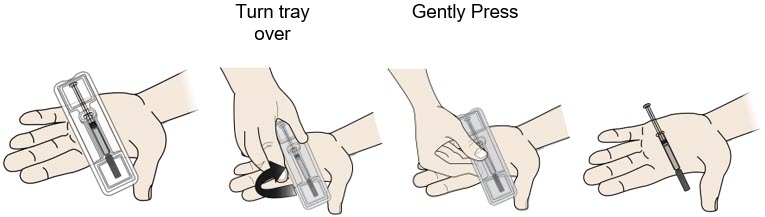
To remove: - Peel paper off of tray.
- Place the tray on your hand.
- Turn the tray over and gently press the middle of the tray's back to release the prefilled syringe into your palm.
- If the prefilled syringe does not release from the tray, gently press on the back of the tray.
Do not pick up or pull the prefilled syringe by the plunger rod or gray needle cap. This could damage the syringe.
Do not remove the gray needle cap from the prefilled syringe until you are ready to inject.
Always hold the prefilled syringe by the syringe barrel.
1 F Check the medicine and syringe. 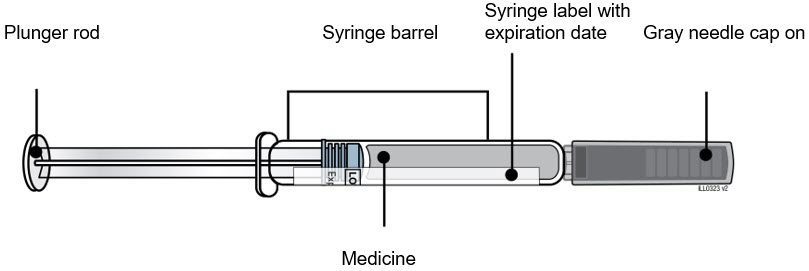
Always hold the prefilled syringe by the syringe barrel.
Check that:- the name REPATHA appears on the prefilled syringe label.
- the medicine in the prefilled syringe is clear and colorless to slightly yellow.
- the expiration date on the prefilled syringe has not passed. If the expiration date has passed, do not use the prefilled syringe.
Do not use the prefilled syringe if any part of the prefilled syringe appears cracked or broken.
Do not use the prefilled syringe if the gray needle cap is missing or not securely attached.
Do not use the prefilled syringe if the medicine is cloudy or discolored or contains particles.
Step 2: Get ready 2 A Carefully pull the gray needle cap straight out and away from your body. Do not leave the gray needle cap off for more than 5 minutes. This can dry out the medicine. 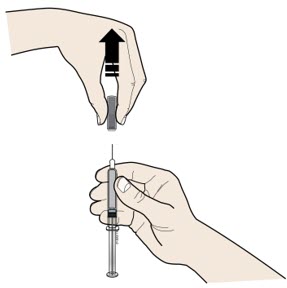
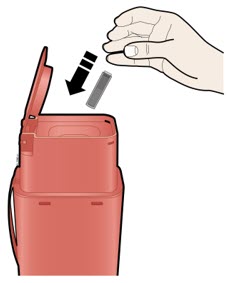
It is normal to see a drop of medicine at the end of the needle. Place the gray needle cap in the sharps disposal container right away. Do not twist or bend the gray needle cap. This can damage the needle. Do not put the gray needle cap back onto the prefilled syringe. Do not try to remove any air bubbles in the syringe before the injection. 2 B Pinch your injection site to create a firm surface. 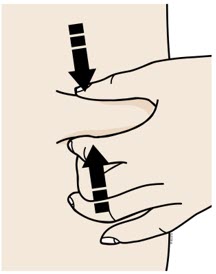
Pinch the skin firmly between your thumb and fingers, creating an area about 2 inches wide. It is important to keep the skin pinched while injecting. Step 3: Inject 3 A Hold the pinch. Insert the needle into the skin using a 45 to 90 degree angle. 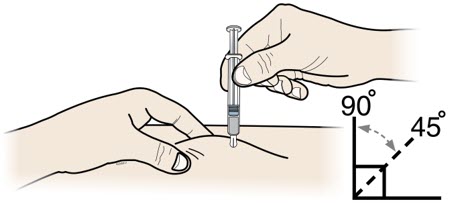
Do not place your finger on the plunger rod while inserting the needle. 3 B Using slow and constant pressure, push the plunger rod all the way down until the prefilled syringe is empty. You may have to push harder on the plunger rod than for other injectable medicines. 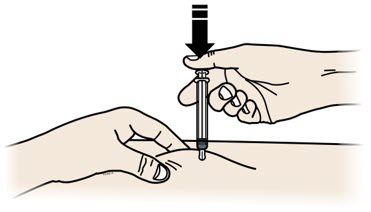
3 C When the prefilled syringe is empty, release your thumb, and gently lift the syringe out of the skin. 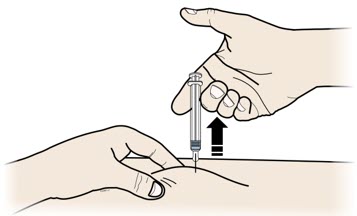
Do not put the gray needle cap back onto the used prefilled syringe. This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Close
Amgen Inc.
Thousand Oaks, CA 91320-1799
U.S. License Number 1080
© 2015-2016, 2021, 2024 Amgen Inc.
All rights reserved.
<part number> Revised: 11/2024 v4 -
INSTRUCTIONS FOR USEInstructions for Use - Repatha® (ri-PAth-a) (evolocumab) prefilled single-dose syringe - Getting to know the prefilled syringe - Before useAfter use - Needle is ...
Instructions for Use
Repatha® (ri-PAth-a)
(evolocumab)
prefilled single-dose syringeGetting to know the prefilled syringe
Before use After use 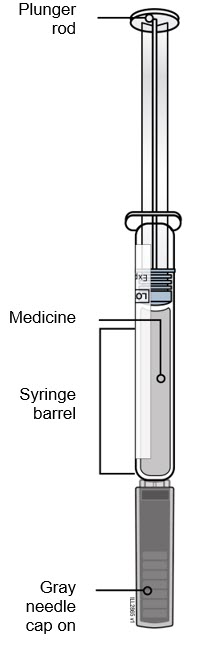
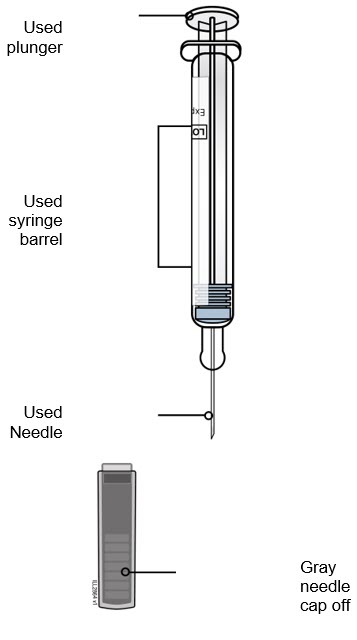
Needle is inside Important Before you use a prefilled single-dose syringe, read this important information: - It is important that you do not try to give the injection until you have fully read and understood this Instructions for Use.
- Check the carton, prefilled syringe label, and prescription to make sure you have the correct medicine and dose.
- Check the expiration date on the REPATHA prefilled syringe carton: do not use if this date has passed.
- It is important that you do not try to give yourself or someone else the injection unless you have received training from your healthcare provider.
- The prefilled syringe is not made with natural rubber latex.
- Keep the prefilled syringe in the original carton to protect from light during storage.
- Keep the prefilled syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- If removed from the refrigerator, the prefilled syringe should be kept at room temperature at 68°F to 77°F (20°C to 25°C) in the original carton and must be used within 30 days. Place the prefilled syringe in a sharps disposal container if it has reached room temperature and has not been used within 30 days.
- Do not freeze the prefilled syringe or use a prefilled syringe that has been frozen.
- Do not store the prefilled syringe in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.
Do not: - Do not use the prefilled syringe if the packaging is open or damaged.
- Do not remove the gray needle cap from the prefilled syringe until you are ready to inject.
- Do not use the prefilled syringe if it has been dropped onto a hard surface. Part of the prefilled syringe may be broken even if you cannot see the break. Use a new prefilled syringe and call 1-844-REPATHA (1-844-737-2842).
- Do not use the prefilled syringe after the expiration date.
A healthcare provider who knows how to use the prefilled syringe should be able to answer your questions. For more information, call 1-844-REPATHA (1-844-737-2842) or visit www.REPATHA.com.
Keep the prefilled syringe and all medicines out of the sight and reach of children.Step 1: Prepare 1 A Remove the prefilled syringe carton from the refrigerator and wait 30 minutes. Wait at least 30 minutes for the prefilled syringe in the carton to reach room temperature before injecting. 
Using the prefilled syringe at room temperature makes sure the full dose is delivered and allows for a more comfortable injection. Do not heat the syringe. Let it warm up on its own naturally. Do not try to warm the prefilled syringe by using a heat source such as hot water or microwave. Do not leave the prefilled syringe in direct sunlight. Do not shake the prefilled syringe. 1 B Gather all materials needed for your injection. Wash your hands thoroughly with soap and water. On a clean, well-lit, flat work surface, place: - 1 REPATHA prefilled syringe in carton
- Alcohol wipes
- Cotton ball or gauze pad
- Adhesive bandage
- Sharps disposal container (see Step 4: Finish)
1 C Choose your injection site. 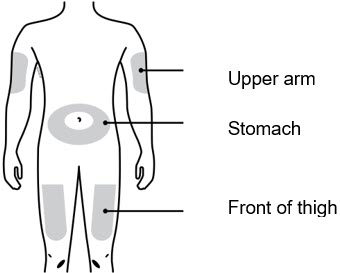
You can use the: - front of your thigh
- stomach (abdomen), except for a 2 inch area around your belly button
Do not choose an area where the skin is tender, bruised, red, or hard. Avoid injecting into areas with scars or stretch marks.
Choose a different site each time you give yourself an injection.1 D Clean your injection site. 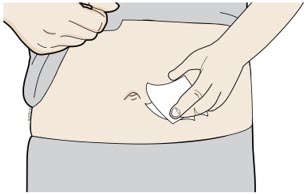
Clean your injection site with an alcohol wipe. Let your skin dry before injecting.
Do not touch this area of skin again before injecting.1 E Remove prefilled syringe from tray. 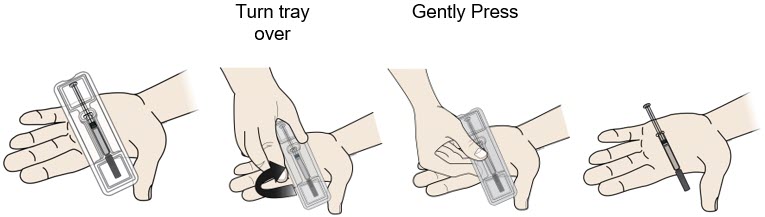
To remove: - Peel paper off of tray.
- Place the tray on your hand.
- Turn the tray over and gently press the middle of the tray's back to release the prefilled syringe into your palm.
- If the prefilled syringe does not release from the tray, gently press on the back of the tray.
Do not pick up or pull the prefilled syringe by the plunger rod or gray needle cap. This could damage the syringe.
Do not remove the gray needle cap from the prefilled syringe until you are ready to inject.
Always hold the prefilled syringe by the syringe barrel.
1 F Check the medicine and syringe. 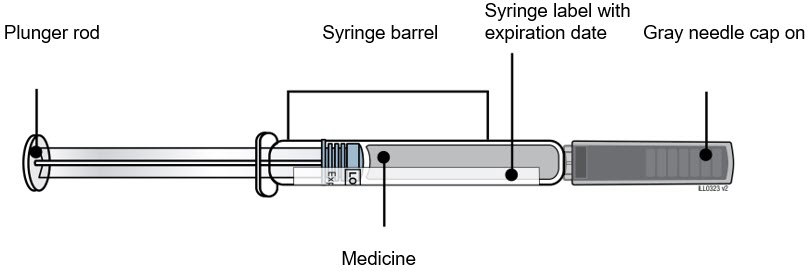
Always hold the prefilled syringe by the syringe barrel.
Check that:- the name REPATHA appears on the prefilled syringe label.
- the medicine in the prefilled syringe is clear and colorless to slightly yellow.
- the expiration date on the prefilled syringe has not passed. If the expiration date has passed, do not use the prefilled syringe.
Do not use the prefilled syringe if any part of the prefilled syringe appears cracked or broken.
Do not use the prefilled syringe if the gray needle cap is missing or not securely attached.
Do not use the prefilled syringe if the medicine is cloudy or discolored or contains particles.
Step 2: Get ready 2 A Carefully pull the gray needle cap straight out and away from your body. Do not leave the gray needle cap off for more than 5 minutes. This can dry out the medicine. 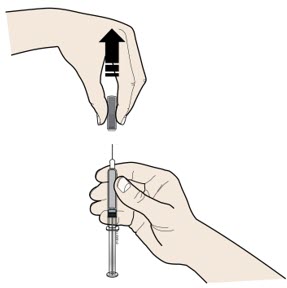
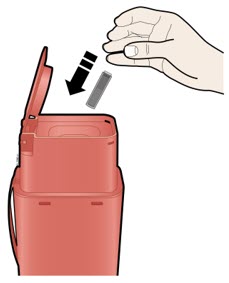
It is normal to see a drop of medicine at the end of the needle. Place the gray needle cap in the sharps disposal container right away. Do not twist or bend the gray needle cap. This can damage the needle. Do not put the gray needle cap back onto the prefilled syringe. Do not try to remove any air bubbles in the syringe before the injection. 2 B Pinch your injection site to create a firm surface. 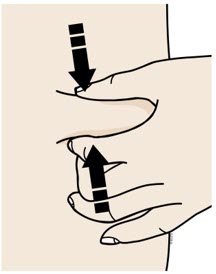
Pinch the skin firmly between your thumb and fingers, creating an area about 2 inches wide. It is important to keep the skin pinched while injecting. Step 3: Inject 3 A Hold the pinch. Insert the needle into the skin using a 45 to 90 degree angle. 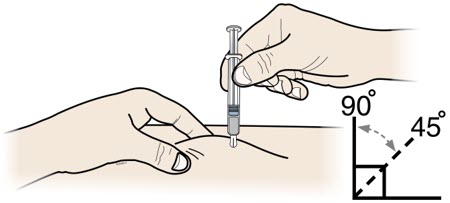
Do not place your finger on the plunger rod while inserting the needle. 3 B Using slow and constant pressure, push the plunger rod all the way down until the prefilled syringe is empty. You may have to push harder on the plunger rod than for other injectable medicines. 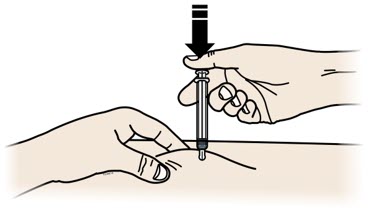
3 C When the prefilled syringe is empty, release your thumb, and gently lift the syringe out of the skin. 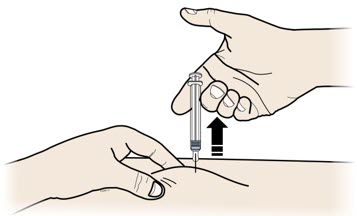
Do not put the gray needle cap back onto the used prefilled syringe. This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Close
Amgen Inc.
Thousand Oaks, CA 91320-1799
U.S. License Number 1080
© 2024 Amgen Inc.
All rights reserved.
<part number> Issued: 11/2024 v1 -
PRINCIPAL DISPLAY PANEL - 140 mg/mL Autoinjector Carton2 x 1 mL Prefilled Autoinjectors - NDC 72511-760-02 - AMGEN® Repatha® SureClick® (evolocumab) Injection - RepathaSupport.com - 140 mg/mL - Prefilled Autoinjector - For Subcutaneous Use Only - Single-Dose ...
2 x 1 mL Prefilled Autoinjectors
NDC 72511-760-02AMGEN®
Repatha® SureClick®
(evolocumab)
InjectionRepathaSupport.com
140 mg/mL
Prefilled AutoinjectorFor Subcutaneous Use Only
Single-Dose Only
Sterile Solution – No Preservative
Store refrigerated at 2°C to 8°C (36°F to 46°F).
Do Not Freeze or Shake.
Store in Carton to Protect from Light.
(see side panel for additional storage information)Keep out of the sight and reach of children.
This Product Contains
Dry Natural Rubber.Do not re-use
CAUTION, See package insert
for full prescribing information
and Instructions for UseRx Only
U.S. License No. 1080
No U.S. standard of potency.Close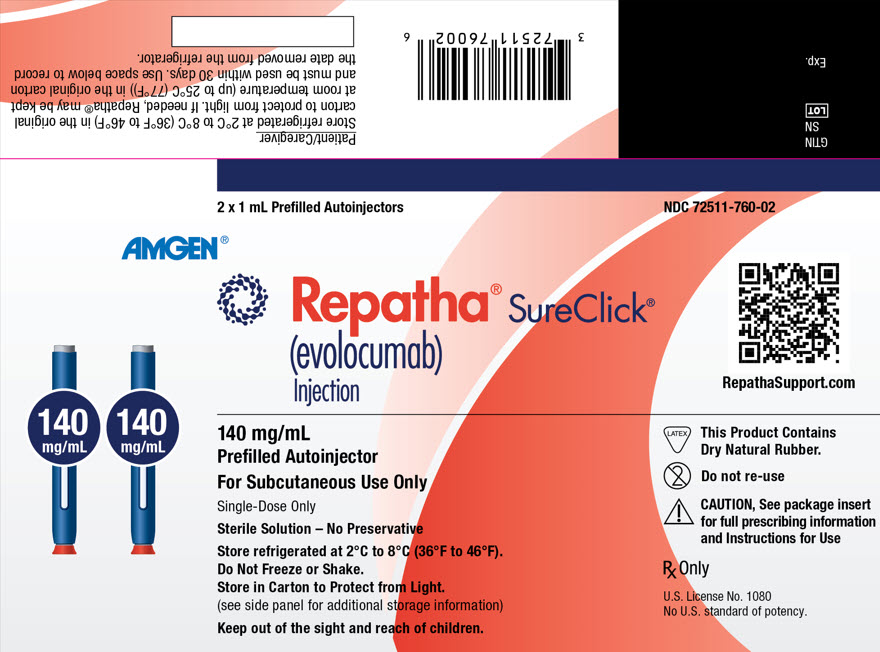
-
PRINCIPAL DISPLAY PANEL - 140 mg/mL Syringe Carton1 x 1 mL Prefilled Syringe - NDC 72511-750-01 - AMGEN® Repatha® (evolocumab) Injection - 140 mg/mL - Prefilled Syringe - For Subcutaneous Use Only - Store refigerated at 2°C to 8°C (36°F to 46°F). Do Not ...
1 x 1 mL Prefilled Syringe
NDC 72511-750-01AMGEN®
Repatha®
(evolocumab)
Injection140 mg/mL
Prefilled SyringeFor Subcutaneous Use Only
Store refigerated at 2°C to 8°C (36°F to 46°F). Do Not Freeze or Shake.
Store in Carton to Protect from Light.
(see side panel for additional storage information)Sterile Solution – No Preservative
Do not re-use
Rx OnlyCAUTION, See package insert for full prescribing
information and Instructions for UseThis Product Contains Dry Natural Rubber.
Keep out of the sight and reach of children
Close
-
PRINCIPAL DISPLAY PANEL - Kit Carton1 x 3.5 mL Prefilled Cartridge - 1 On-Body Infusor - NDC 72511-770-01 - AMGEN® Repatha® Pushtronex® system - (evolocumab) On-Body Infusor and Prefilled Cartridge - 420 mg/3.5 mL - For Subcutaneous Use ...
1 x 3.5 mL Prefilled Cartridge
1 On-Body Infusor
NDC 72511-770-01AMGEN®
Repatha® Pushtronex® system
(evolocumab)
On-Body Infusor and Prefilled Cartridge420 mg/3.5 mL
For Subcutaneous Use Only
Single-Dose Only
Sterile Solution – No Preservative
Store refrigerated at 2°C to 8°C (36°F to 46°F).
Do Not Freeze or Shake.
Store in Carton to Protect from Light.
(see side panel for additional storage information)Keep out of the sight and reach of children.
Refer to Instructions for Use
Do Not Use if Package is Damaged
On-Body Infusor Sterilized
Using Ethylene Oxide420 mg/
3.5 mLMR Unsafe
Keep Dry
Do not re-use
Type BF Applied Part
Relative Humidity
Range is 15% to 85%Rx Only
Close
-
PRINCIPAL DISPLAY PANEL - 1 mL Autoinjector Carton1 x 1 mL Prefilled Autoinjector - NDC 72511-393-01 - AMGEN® 140 - mg/mL - Repatha® SureClick® (evolocumab) Injection - RepathaSupport.com - 140 mg/mL - Prefilled Autoinjector - For Subcutaneous Use ...
1 x 1 mL Prefilled Autoinjector
NDC 72511-393-01AMGEN®
140
mg/mLRepatha® SureClick®
(evolocumab)
InjectionRepathaSupport.com
140 mg/mL
Prefilled AutoinjectorFor Subcutaneous Use Only
Single-Dose Only
Sterile Solution – No Preservative
Store refrigerated at 2°C to 8°C (36°F to 46°F). Do Not Freeze or Shake.
Store in Carton to Protect from Light.
(see side panel for additional storage information)Keep out of the sight and reach of children.
Not made
with natural
rubber latexDo not re-use
CAUTION, See package insert
for full prescribing information
and Instructions for UseRx Only
U.S. License No. 1080
No U.S. standard of potency.Close
-
PRINCIPAL DISPLAY PANEL - 2 Autoinjector Carton2 x 1 mL Prefilled Autoinjectors - NDC 72511-393-02 - AMGEN® 140 - mg/mL - 140 - mg/mL - Repatha® SureClick® (evolocumab) Injection - RepathaSupport.com - 140 mg/mL - Prefilled Autoinjector - For Subcutaneous Use ...
2 x 1 mL Prefilled Autoinjectors
NDC 72511-393-02AMGEN®
140
mg/mL140
mg/mLRepatha® SureClick®
(evolocumab)
InjectionRepathaSupport.com
140 mg/mL
Prefilled AutoinjectorFor Subcutaneous Use Only
Single-Dose Only
Sterile Solution – No Preservative
Store refrigerated at 2°C to 8°C (36°F to 46°F).
Do Not Freeze or Shake.
Store in Carton to Protect from Light.
(see side panel for additional storage information)Keep out of the sight and reach of children.
Not made
with natural
rubber latexDo not re-use
CAUTION, See package insert
for full prescribing information
and Instructions for UseRx Only
U.S. License No. 1080
No U.S. standard of potency.Close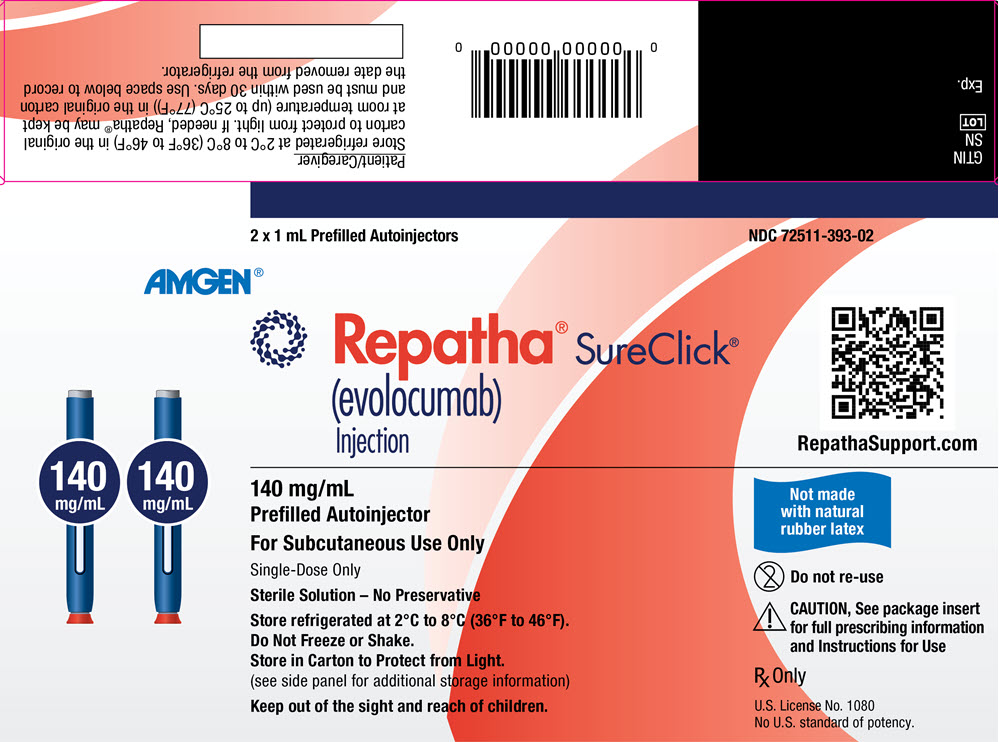
-
PRINCIPAL DISPLAY PANEL - 140 mg/mL Syringe Carton - NDC 72511-501-011 x 1 mL Prefilled Syringe - NDC 72511-501-01 - AMGEN® 140 - mg/mL - Repatha® (evolocumab) Injection - Not made - with natural - rubber latex - 140 mg/mL - Prefilled Syringe - For Subcutaneous Use Only - Store ...
1 x 1 mL Prefilled Syringe
NDC 72511-501-01AMGEN®
140
mg/mLRepatha®
(evolocumab)
InjectionNot made
with natural
rubber latex140 mg/mL
Prefilled SyringeFor Subcutaneous Use Only
Store refrigerated at 2°C to 8°C (36°F to 46°F). Do Not Freeze or Shake.
Store in Carton to Protect from Light.
(see side panel for additional storage information)Sterile Solution – No Preservative
Rx Only
Do not re-use
CAUTION, See package insert for full prescribing
information and Instructions for UseKeep out of the sight and reach of children
GTIN
SN
LOT
Exp.Close
-
INGREDIENTS AND APPEARANCEProduct Information
REPATHA evolocumab injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72511-760 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EVOLOCUMAB (UNII: LKC0U3A8NJ) (EVOLOCUMAB - UNII:LKC0U3A8NJ) EVOLOCUMAB 140 mg in 1 mL Inactive Ingredients Ingredient Name Strength PROLINE (UNII: 9DLQ4CIU6V) 25 mg in 1 mL ACETATE ION (UNII: 569DQM74SC) 1.2 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.1 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72511-760-02 2 in 1 CARTON 10/09/2018 1 NDC:72511-760-01 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:72511-760-91 1 in 1 CARTON 06/01/2022 2 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125522 10/09/2018 REPATHA evolocumab injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72511-750 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EVOLOCUMAB (UNII: LKC0U3A8NJ) (EVOLOCUMAB - UNII:LKC0U3A8NJ) EVOLOCUMAB 140 mg in 1 mL Inactive Ingredients Ingredient Name Strength PROLINE (UNII: 9DLQ4CIU6V) 25 mg in 1 mL ACETATE ION (UNII: 569DQM74SC) 1.2 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.1 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72511-750-01 1 in 1 CARTON 10/09/2018 1 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125522 10/09/2018 REPATHA evolocumab kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72511-770 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72511-770-01 1 in 1 CARTON 10/09/2018 2 NDC:72511-770-91 1 in 1 CARTON 08/22/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 CARTRIDGE 3.5 mL Part 1 of 1 REPATHA evolocumab injection, solution Product Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EVOLOCUMAB (UNII: LKC0U3A8NJ) (EVOLOCUMAB - UNII:LKC0U3A8NJ) EVOLOCUMAB 420 mg in 3.5 mL Inactive Ingredients Ingredient Name Strength PROLINE (UNII: 9DLQ4CIU6V) 89 mg in 3.5 mL ACETATE ION (UNII: 569DQM74SC) 4.2 mg in 3.5 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.35 mg in 3.5 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 TRAY 1 3.5 mL in 1 CARTRIDGE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125522 10/09/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125522 10/09/2018 REPATHA evolocumab injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72511-393 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EVOLOCUMAB (UNII: LKC0U3A8NJ) (EVOLOCUMAB - UNII:LKC0U3A8NJ) EVOLOCUMAB 140 mg in 1 mL Inactive Ingredients Ingredient Name Strength PROLINE (UNII: 9DLQ4CIU6V) 25 mg in 1 mL ACETATE ION (UNII: 569DQM74SC) 1.2 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.1 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72511-393-02 2 in 1 CARTON 11/24/2024 1 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:72511-393-01 1 in 1 CARTON 11/24/2024 2 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125522 11/24/2024 REPATHA evolocumab injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72511-501 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EVOLOCUMAB (UNII: LKC0U3A8NJ) (EVOLOCUMAB - UNII:LKC0U3A8NJ) EVOLOCUMAB 140 mg in 1 mL Inactive Ingredients Ingredient Name Strength PROLINE (UNII: 9DLQ4CIU6V) 25 mg in 1 mL ACETATE ION (UNII: 569DQM74SC) 1.2 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.1 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72511-501-01 1 in 1 CARTON 11/24/2024 1 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125522 11/24/2024 Labeler - Amgen USA Inc. (962075045)
CloseRegistrant - Amgen, Inc (039976196)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
REPATHA- evolocumab injection, solution
REPATHA- evolocumab kit
Number of versions: 20
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Mar 3, 2025 | 23 (current) | download |
| Dec 6, 2024 | 22 | download |
| Sep 4, 2024 | 21 | download |
| Feb 5, 2024 | 20 | download |
| Jun 19, 2023 | 19 | download |
| May 9, 2023 | 18 | download |
| Oct 10, 2022 | 17 | download |
| Sep 5, 2022 | 16 | download |
| Jul 20, 2022 | 15 | download |
| Oct 12, 2021 | 13 | download |
| May 20, 2021 | 12 | download |
| Mar 15, 2021 | 11 | download |
| Jun 9, 2020 | 10 | download |
| May 28, 2020 | 9 | download |
| May 21, 2020 | 8 | download |
| Sep 16, 2019 | 7 | download |
| Apr 25, 2019 | 5 | download |
| Mar 1, 2019 | 4 | download |
| Nov 1, 2018 | 2 | download |
| Oct 11, 2018 | 1 | download |
RxNorm
REPATHA- evolocumab injection, solution
REPATHA- evolocumab kit
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 1665895 | evolocumab 140 MG in 1 ML Auto-Injector | PSN |
| 2 | 1665895 | 1 ML evolocumab 140 MG/ML Auto-Injector | SCD |
| 3 | 1665900 | Repatha 140 MG in 1 ML Auto-Injector | PSN |
| 4 | 1665900 | 1 ML evolocumab 140 MG/ML Auto-Injector [Repatha] | SBD |
| 5 | 1665900 | 1 ML Repatha 140 MG/ML Auto-Injector | SY |
| 6 | 1665900 | 1 ML Repatha SureClick 140 MG/ML Auto-Injector | SY |
| 7 | 1665904 | evolocumab 140 MG in 1 ML Prefilled Syringe | PSN |
| 8 | 1665904 | 1 ML evolocumab 140 MG/ML Prefilled Syringe | SCD |
| 9 | 1665906 | Repatha 140 MG in 1 ML Prefilled Syringe | PSN |
| 10 | 1665906 | 1 ML evolocumab 140 MG/ML Prefilled Syringe [Repatha] | SBD |
| 11 | 1665906 | 1 ML Repatha 140 MG/ML Prefilled Syringe | SY |
| 12 | 1801319 | evolocumab 420 in 3.5 ML Cartridge | PSN |
| 13 | 1801319 | 3.5 ML evolocumab 120 MG/ML Cartridge | SCD |
| 14 | 1801319 | evolocumab 420 per 3.5 ML Cartridge | SY |
| 15 | 1801322 | Repatha 420 MG in 3.5 ML Cartridge | PSN |
| 16 | 1801322 | 3.5 ML evolocumab 120 MG/ML Cartridge [Repatha] | SBD |
| 17 | 1801322 | 3.5 ML Repatha 120 MG/ML Cartridge | SY |
| 18 | 1801322 | Repatha 420 MG per 3.5 ML Cartridge | SY |
Get Label RSS Feed for this Drug
REPATHA- evolocumab injection, solution
REPATHA- evolocumab kit
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=cd61e902-166d-4aa6-9f3c-a18c1008d07e
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
REPATHA- evolocumab injection, solution
REPATHA- evolocumab kit
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 72511-393-01 |
| 2 | 72511-393-02 |
| 3 | 72511-501-01 |
| 4 | 72511-750-01 |
| 5 | 72511-760-01 |
| 6 | 72511-760-02 |
| 7 | 72511-760-91 |
| 8 | 72511-770-01 |
| 9 | 72511-770-91 |