Label: DOXYCYCLINE HYCLATE tablet, delayed release
- NDC Code(s): 68308-710-10, 68308-715-10, 68308-715-30, 68308-716-30, view more
- Packager: Mayne Pharma Commercial LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated April 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DOXYCYCLINE HYCLATE DELAYED-RELEASE TABLETS safely and effectively. See full prescribing information for DOXYCYCLINE HYCLATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Rickettsial Infections - Doxycycline hyclate delayed-release tablets are indicated for treatment of Rocky Mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsialpox ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage and Administration Instructions - Doxycycline hyclate delayed-release tablets are not substitutable on a mg per mg basis with other oral doxycyclines. To avoid prescribing ...
-
3 DOSAGE FORMS AND STRENGTHSDoxycycline hyclate delayed-release tablets, USP 80 mg are white, oval scored tablets containing yellow pellets and debossed with "D|8" on one face and plain on the other. Each tablet contains ...
-
4 CONTRAINDICATIONSDoxycycline hyclate delayed-release tablets are contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
-
5 WARNINGS AND PRECAUTIONS5.1 Tooth Development - The use of drugs of the tetracycline-class during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration ...
-
6 ADVERSE REACTIONS6.1 Clinical Trial Experience - The safety and efficacy of doxycycline hyclate delayed-release tablets, 200 mg as a single daily dose was evaluated in a multicenter, randomized, double-blind ...
-
7 DRUG INTERACTIONS7.1 Anticoagulant Drugs - Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies on the use of doxycycline in pregnant women. The vast majority of reported experience with doxycycline during ...
-
10 OVERDOSAGEIn case of overdosage, discontinue medication, treat symptomatically and institute supportive measures. Dialysis does not alter serum half-life and thus would not be of benefit in treating cases ...
-
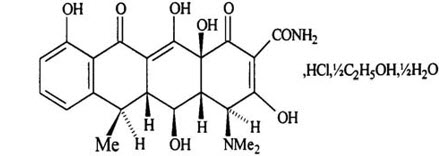
11 DESCRIPTIONDoxycycline hyclate delayed-release tablets, contain specially coated pellets of doxycycline hyclate, a tetracycline class drug synthetically derived from oxytetracycline, in a delayed-release ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Doxycycline is a tetracycline-class antimicrobial drug [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Following single and multiple-dose administration of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to evaluate carcinogenic potential of doxycycline have not been conducted. However, there has been ...
-
14 CLINICAL STUDIESThis was a randomized, double-blind, active-controlled, multicenter trial which enrolled 495 subjects, between 19 to 45 years of age with a confirmed diagnosis of urogenital C. trachomatis ...
-
15 REFERENCESFriedman JM, Polifka JE. Teratogenic Effects of Drugs. A Resource for Clinicians (TERIS). Baltimore, MD: The Johns Hopkins University Press: 2000: 149-195. Cziezel AE and Rockenbauer M ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGDoxycycline hyclate delayed-release tablets, USP, 80 mg are white, oval scored tablets containing yellow pellets and debossed with "D|8" on one face and plain on the other. Each tablet contains ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients taking doxycycline for malaria prophylaxis: that no present-day antimalarial agent, including doxycycline, guarantees protection against malaria. to avoid being bitten by ...
-
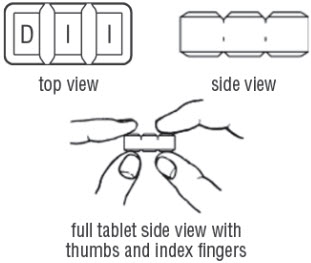
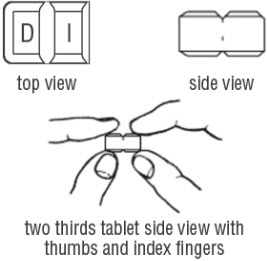
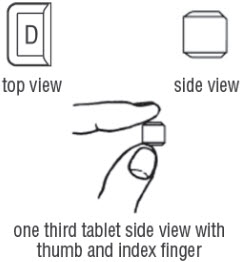
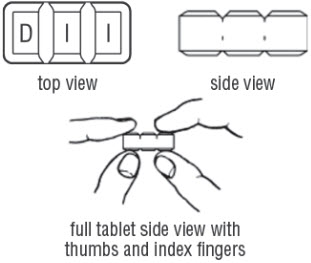
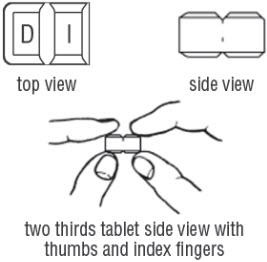
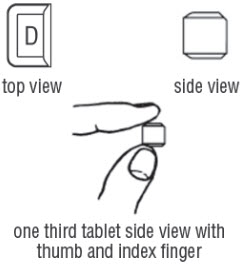
FDA-Approved Patient LabelingDoxycycline hyclate delayed-release tablets, 50 mg, 75 mg, 80 mg, 100 mg, 150 mg and 200 mgInstructions for Breaking the 150 mg Doxycycline hyclate delayed-release dual-scored tabletYour doctor may find it necessary to adjust your dosage of doxycycline hyclate delayed-release tablets to obtain the proper treatment response. The tablet is marked with separation lines (score ...
-
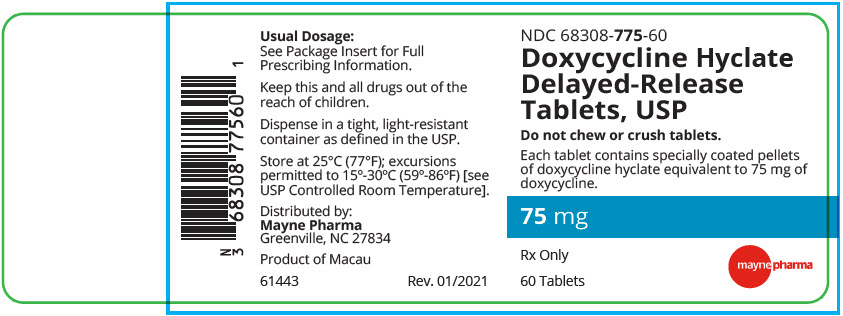
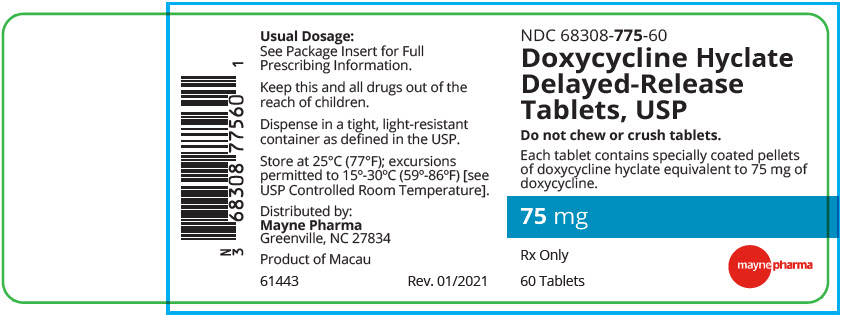
PRINCIPAL DISPLAY PANEL - 75 mg Tablet Bottle LabelNDC 68308-775-60 - Doxycycline Hyclate - Delayed-Release - Tablets, USP - Do not chew or crush tablets. Each tablet contains specially coated pellets - of doxycycline hyclate equivalent to 75 mg ...
-
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle LabelNDC 68308-710-10 - Doxycycline Hyclate - Delayed-Release - Tablets, USP - Do not chew or crush tablets. Each tablet contains specially coated pellets of - doxycycline hyclate equivalent to 100 mg of ...
-
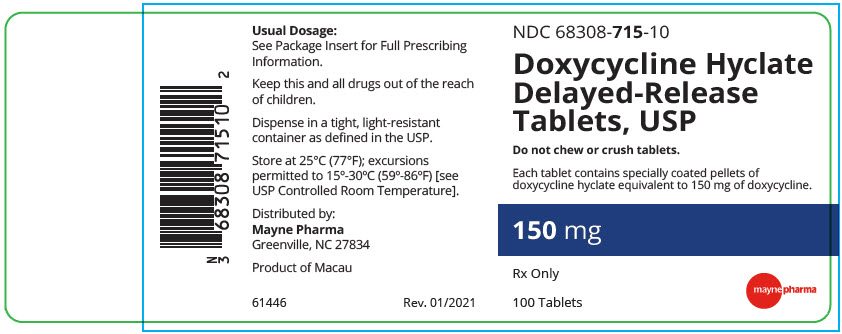
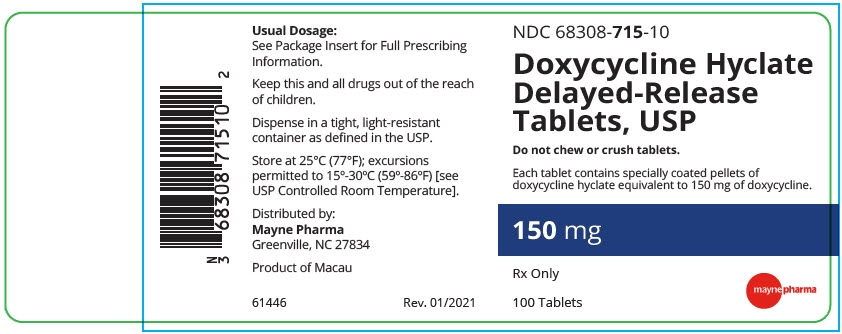
PRINCIPAL DISPLAY PANEL - 150 mg Tablet Bottle LabelNDC 68308-715-10 - Doxycycline Hyclate - Delayed-Release - Tablets, USP - Do not chew or crush tablets. Each tablet contains specially coated pellets of - doxycycline hyclate equivalent to 150 mg of ...
-
PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle LabelNDC 68308-716-30 - Doxycycline Hyclate - Delayed-Release - Tablets, USP - Do not chew or crush tablets. Each tablet contains specially coated pellets of - doxycycline hyclate equivalent to 200 mg of ...
-
INGREDIENTS AND APPEARANCEProduct Information