Label: WALGREENS PAIN BURN ITCH RELIEF- benzocaine 20%, menthol 0.5% spray
- NDC Code(s): 0363-2053-02
- Packager: Walgreens
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
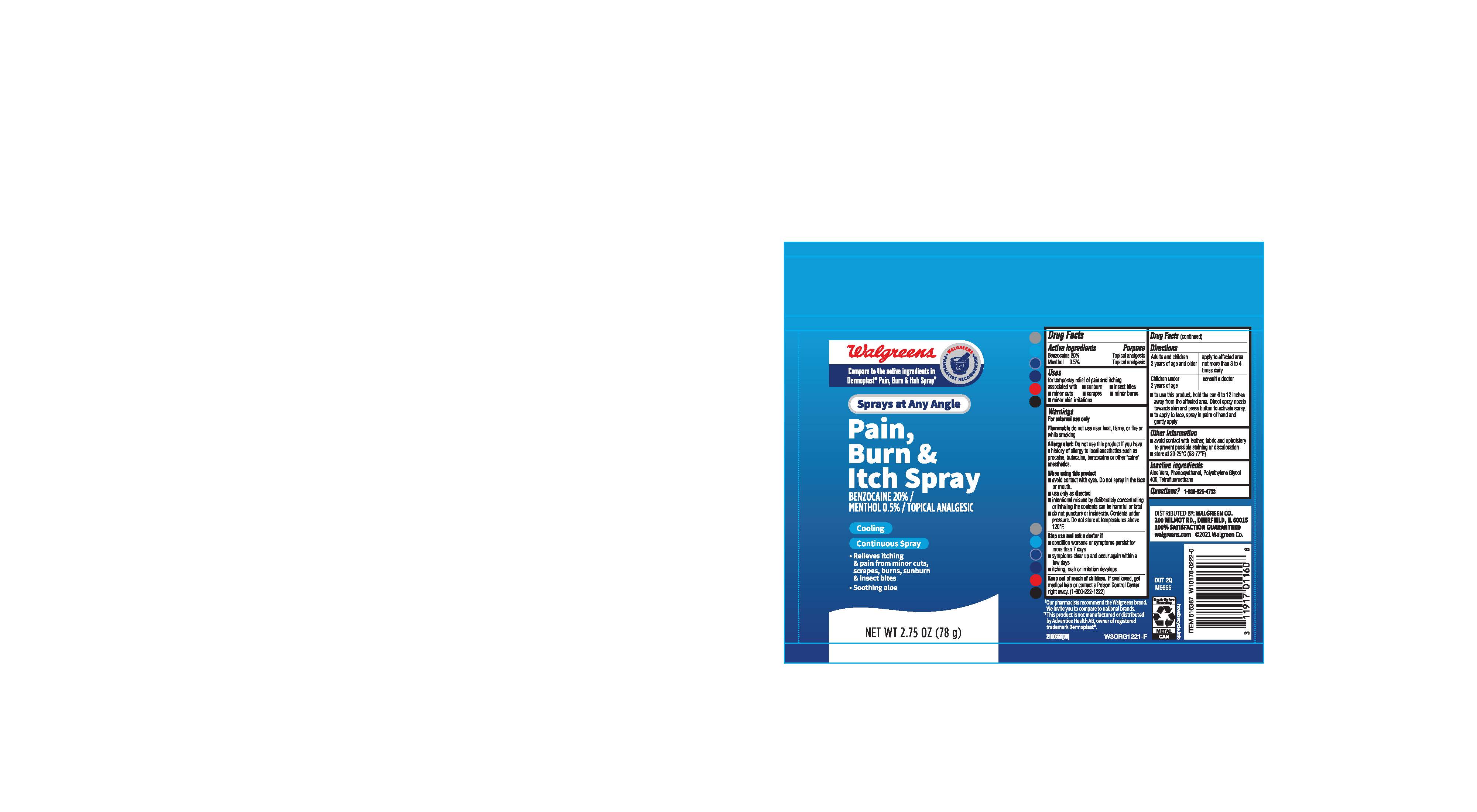
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Adults and children 2 years of age and older : Apply to the affected area not more than 3 to 4 times daily
Children under 2 years of age: Cinsult a doctor
- To use this product, hold the can 6 to 12 inches away from the affected area. Direct spray nozzle towards skin and press button to activate spray.
- To apply to face, spray in palm of hand and gently apply.
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WALGREENS PAIN BURN ITCH RELIEF
benzocaine 20%, menthol 0.5% sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-2053 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 mg in 1 g BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 200 mg in 1 g Inactive Ingredients Ingredient Name Strength PHENOXYETHANOL (UNII: HIE492ZZ3T) ALOE VERA LEAF (UNII: ZY81Z83H0X) NORFLURANE (UNII: DH9E53K1Y8) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-2053-02 78 g in 1 CAN; Type 0: Not a Combination Product 05/05/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/05/2022 Labeler - Walgreens (008965063)