Label: VALACYCLOVIR tablet, film coated
- NDC Code(s): 61919-066-20, 61919-066-60
- Packager: Direct Rx
- This is a repackaged label.
- Source NDC Code(s): 59746-324
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 29, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
INDICATIONS AND USAGE SECTION 1.1 Adult Patients - Cold Sores (Herpes Labialis): Valacyclovir Tablets, USP are indicated for treatment of cold sores (herpes labialis). The efficacy of valacyclovir hydrochloride initiated after ...
-
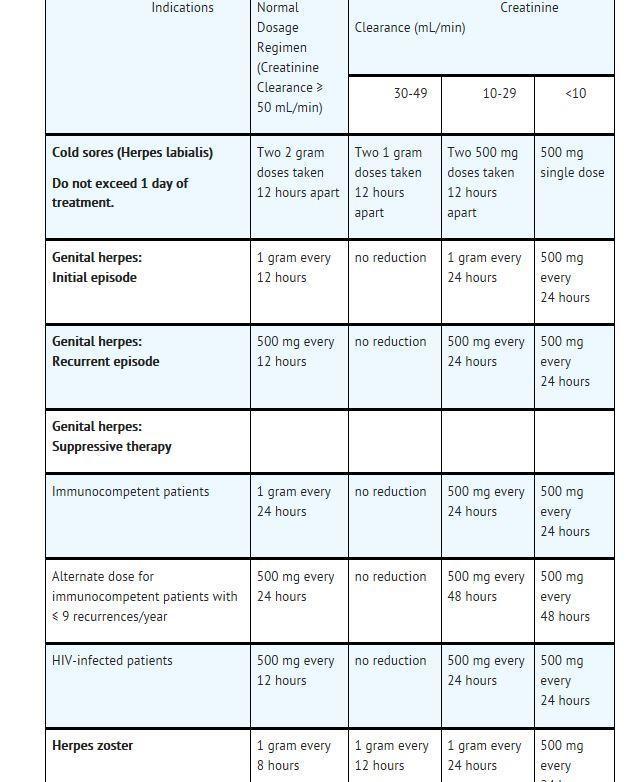
DOSAGE AND ADMINISTRATION USAGE DOSAGE AND ADMINISTRATION - Valacyclovir tablets, USP may be given without regard to meals. Valacyclovir oral suspension (25 mg/mLor 50 mg/mL) may be prepared extemporaneously from valacyclovir ...
-
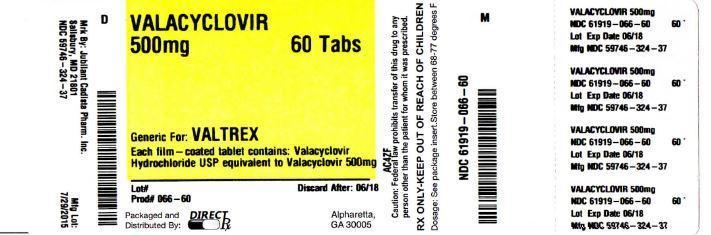
DOSAGE FORMS AND STRENGTHS Valacyclovir Tablets,USP: 500 mg: blue colored, capsule shaped, film coated tablets debossed with "C324 500" on one side and plain on the other side. 1 gram: blue ...
-
CONTRAINDICATIONS SECTION Valacyclovir hydrochloride is contraindicated in patients who have had a demonstrated clinically significant hypersensitivity reaction (e.g., anaphylaxis) to valacyclovir, acyclovir, or any ...
-
WARNINGS AND PRECAUTIONS SECTION 5.1 Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome (TTP/HUS) TTP/HUS, in some cases resulting in death, has occurred in patients with advanced HIV disease and also in allogeneic ...
-
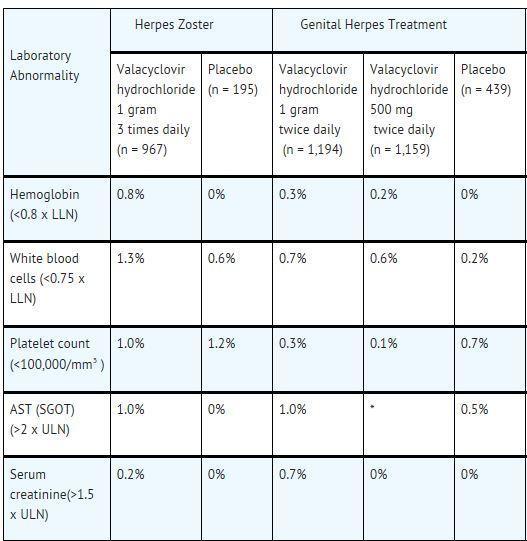
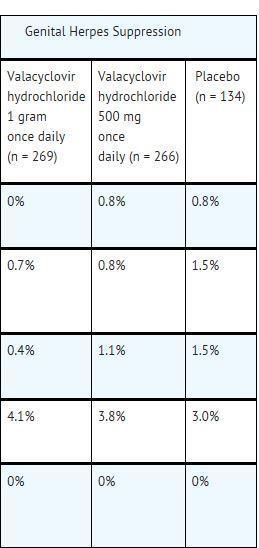
ADVERSE REACTIONS SECTION The following serious adverse reactions are discussed in greater detail in other sections of the labeling: Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome [see Warnings ...
-
DRUG INTERACTIONSNo clinically significant drug-drug or drug-food interactions with valacyclovir hydrochloride are known [see Clinical Pharmacology (12.3)].
-
USE IN SPECIFIC POPULATIONS SECTION 8.1 Pregnancy - Pregnancy Category B. There are no adequate and well-controlled studies of valacyclovir hydrochloride or acyclovir in pregnant women. Based on prospective pregnancy registry data on ...
-
OVERDOSAGE SECTION Caution should be exercised to prevent inadvertent overdose [see Use in Specific Populations (8.5), (8.6)]. Precipitation of acyclovir in renal tubules may occur when the solubility (2.5 mg/mL) is ...
-
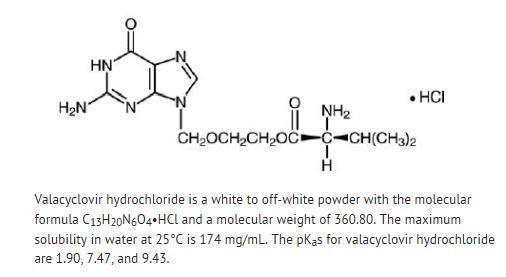
DESCRIPTION SECTION Valacyclovir hydrochloride,USP is the hydrochloride salt of the L-valyl ester of the antiviral drug acyclovir. Valacyclovir tablets, USP are for oral administration. Each tablet contains ...
-
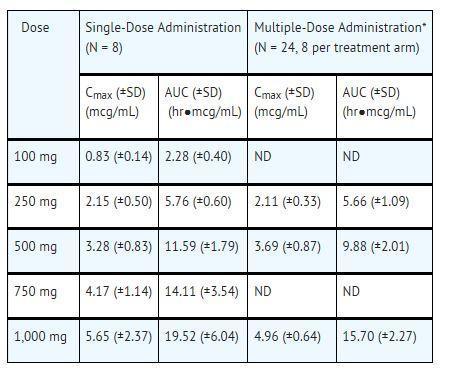
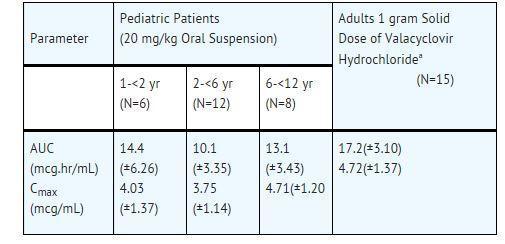
CLINICAL PHARMACOLOGY SECTION 12.1 Mechanism of Action - Valacyclovir is an antiviral drug [see Clinical Pharmacology (12.4)]. 12.3 Pharmacokinetics - The pharmacokinetics of valacyclovir and acyclovir after oral administration of ...
-
NONCLINICAL TOXICOLOGY SECTION 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - The data presented below include references to the steady-state acyclovir AUC observed in humans treated with 1 gram valacyclovir ...
-
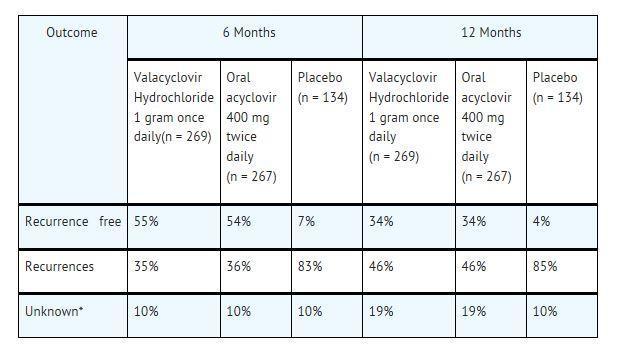
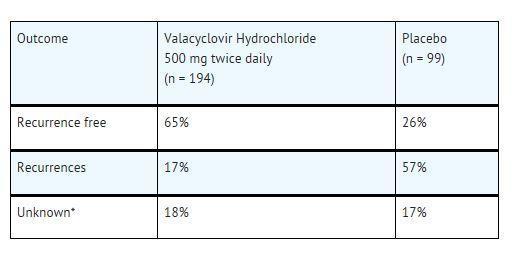
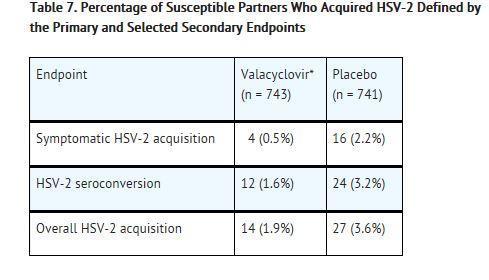
CLINICAL STUDIES SECTION 14.1 Cold Sores (Herpes Labialis) Two double-blind, placebo-controlled clinical trials were conducted in 1,856 healthy adults and adolescents (≥12 years old) with a history of recurrent cold ...
-
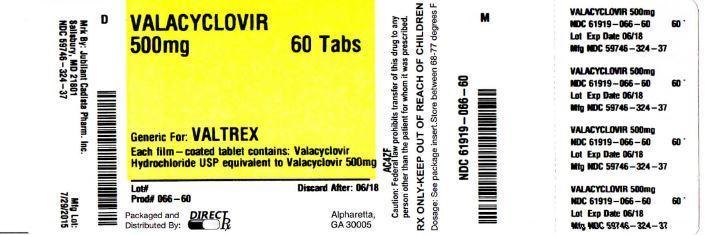
HOW SUPPLIED SECTIONValacyclovir tablets, USP 500 mg (blue colored, capsule shaped, film coated tablets debossed with "C324 500" on one side and plain on the other side) containing valacyclovir hydrochloride ...
-
PATIENT MEDICATION INFORMATION SECTION See FDA-Approved Patient Labeling (17.6). 17.1 Importance of Adequate Hydration - Patients should be advised to maintain adequate hydration. 17.2 Cold Sores (Herpes Labialis) Patients should be ...
-
INFORMATION FOR PATIENTS SECTION VALACYCLOVIR TABLETS USP - Read the Patient Information that comes with valacyclovir tablets,USP before you start using it and each time you get a refill. There may be new information. This ...
-
PACKAGEL LABEL
-
PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information