Label: REVOLUTION PLUS- selamectin and sarolaner solution
-
NDC Code(s):
54771-0411-1,
54771-0411-2,
54771-0411-3,
54771-0412-1, view more54771-0412-2, 54771-0412-3, 54771-0416-1, 54771-0416-2, 54771-0416-3
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION:
-
DESCRIPTION:
REVOLUTION PLUS (selamectin and sarolaner topical solution) for Cats is available as a colorless to yellow, ready to use solution in single dose tubes for topical (dermal) treatment of cats and kittens eight weeks of age and older. The content of each tube is formulated to provide a minimum of 2.7 mg/lb (6 mg/kg) of body weight of selamectin and 0.45 mg/lb (1.0 mg/kg) of body weight of sarolaner. Inactive ingredients: Dipropylene Glycol Monomethylether, Isopropyl Alcohol, Butylated Hydroxytoluene.
Selamectin is a member of the macrocyclic lactone class of parasiticides and the chemical name is 25-Cyclohexyl-4’-O-de(2,6-dideoxy-3-O-methyl-α-l-arabino-hexopyranosyl)-5-demethoxy-25-de(1-methylpropyl)-22,23-dihydro-5-(hydroxyimino)-avermectin A1a.
Sarolaner is a member of the isoxazoline class of parasiticides and the chemical name is 1(5’-((5S)-5-(3,5-Dichloro-4-fluorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl)-3’-H-spiro(azetidine-3,1’-(2)benzofuran)-1-yl)-2-(methylsulfonyl)ethanone. REVOLUTION PLUS contains the S-enantiomer of sarolaner.
-
INDICATIONS:
REVOLUTION PLUS is indicated for the prevention of heartworm disease caused by Dirofilaria immitis, the treatment and control of roundworm (Toxocara cati) and intestinal hookworm (Ancylostoma tubaeforme) infections, and the treatment and control of ear mite (Otodectes cynotis) infestations.
REVOLUTION PLUS kills adult fleas (Ctenocephalides felis) and is indicated for the treatment and prevention of flea infestations, the prevention of Dipylidium caninum (tapeworm) infections as a direct result of killing Ctenocephalides felis vector fleas on the treated cat, and the treatment and control of tick infestations with Amblyomma americanum (lone star tick), Amblyomma maculatum (Gulf Coast tick), Dermacentor variabilis (American dog tick), and Ixodes scapularis (black-legged tick) for one month in cats and kittens 8 weeks and older, and weighing 2.8 pounds or greater. -
DOSAGE AND ADMINISTRATION:
The recommended minimum dosage is 2.7 mg selamectin per pound (6 mg/kg) of body weight and 0.45 mg sarolaner per pound (1 mg/kg) of body weight.
Administer the entire contents of a single tube (or two tubes in combination for cats weighing over 22 pounds) of REVOLUTION PLUS topically in accordance with the following table.
Body Weight (lbs) Tube Cap Color Tube Volume (mL) Tube Contents Selamectin
(mg/tube)
Sarolaner
(mg/tube)
2.8 - 5.5 gold
0.25
15 2.5 5.6 - 11 orange 0.5 30 5 11.1 - 22* green 1 60 10 * Cats over 22 lbs should be treated with the appropriate combination of tubes.
Recommended for use in cats and kittens 8 weeks of age or older.A veterinarian or veterinary technician should demonstrate or instruct the pet owner regarding the appropriate technique for applying REVOLUTION PLUS topically to cats and kittens prior to first use.
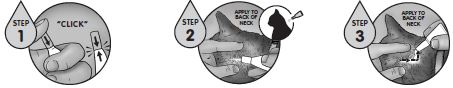
Firmly press the cap down to puncture the seal on the REVOLUTION PLUS tube; a clicking sound will confirm that the cap has successfully punctured the seal. Remove the cap and check to ensure that the tip of the tube is open. To administer the product, part the hair on the back of the animal at the base of the neck in front of the shoulder blades until the skin is visible. Place the tip of the tube on the skin and squeeze the tube 3 or 4 times to empty its entire contents directly on to the skin in one spot. Keeping the tube squeezed, drag it away from the liquid and lift to remove. Check the tube to ensure that it is empty.
Do not massage the product into the skin. Due to alcohol content, do not apply to broken skin. Avoid contact between the product and fingers. Do not apply when the hair coat is wet. Stiff hair, clumping of hair, hair discoloration, or a slight powdery residue may be observed at the treatment site in some cats. These effects are temporary and do not affect the safety or effectiveness of the product. Discard empty tubes in your ordinary household refuse.
The effectiveness of REVOLUTION PLUS against D. immitis after bathing has not been evaluated. Bathing or shampooing the cat 24 hours after treatment does not reduce the effectiveness of topical selamectin, one of the ingredients of REVOLUTION PLUS, against D. immitis.
Flea Treatment and Prevention
For the treatment and prevention of flea infestations, REVOLUTION PLUS may begin at any time of year. REVOLUTION PLUS should be administered year-round at monthly intervals or begin at least one month before fleas become active. If a cat is already infested with fleas when the first dose of REVOLUTION PLUS is administered, adult fleas on the animal are killed before they can lay eggs. However, an environmental infestation may persist for a short time after beginning treatment with REVOLUTION PLUS because of the development of adult fleas from eggs that were laid prior to the initiation of treatment.
Tapeworm Prevention
For the prevention of Dipylidium caninum (tapeworm) infections as a direct result of killing Ctenocephalides felis vector fleas, REVOLUTION PLUS may begin at any time of year. REVOLUTION PLUS should be administered year-round at monthly intervals or begin at least one month before the fleas become active.
REVOLUTION PLUS will only prevent D. caninum infections by killing the fleas on the treated cat. Cats may also become infected with D. caninum by ingesting fleas from untreated animals, it is therefore recommended that all pets in a household are treated for fleas.Tick Treatment and Control
For the treatment and control of infestations with Ixodes scapularis, Amblyomma maculatum, Dermacentor variabilis, and Amblyomma americanum, REVOLUTION PLUS may begin at any time of year. REVOLUTION PLUS should be administered year-round at monthly intervals or begin at least one month before the ticks become active.
Heartworm Prevention
For the prevention of heartworm disease, REVOLUTION PLUS must be administered on a monthly basis. REVOLUTION PLUS should be administered year-round or at least within one month after the animal’s first exposure to mosquitoes and monthly thereafter until the end of the mosquito season. The final dose must be given within one month after the last exposure to mosquitoes. If a dose is missed and a monthly interval between dosing is exceeded then immediate administration of REVOLUTION PLUS and resumption of monthly dosing will minimize the opportunity for the development of adult heartworms. When replacing another monthly heartworm preventive product in a heartworm disease prevention program, the first dose of REVOLUTION PLUS must be given within a month of the last dose of the former medication.
Selamectin, one of the active ingredients in REVOLUTION PLUS, is a macrocyclic lactone compound. These compounds effectively prevent the development of adult heartworms when administered to cats within one month of exposure to infective (L3) Dirofilaria immitis larvae. This product effectively eliminates the tissue stage of heartworm larvae for a month after infection. Cats with unknown heartworm history that test negative prior to the initiation of REVOLUTION PLUS may be harboring pre-patent infections at the time REVOLUTION PLUS was started. Testing such animals 3-4 months after initiation of REVOLUTION PLUS would be necessary to confirm their negative heartworm status.
At the discretion of the veterinarian, cats ≥ 6 months of age may be tested to determine the presence of existing heartworm infections before beginning treatment with REVOLUTION PLUS. Cats already infected with adult heartworms can be given REVOLUTION PLUS monthly to prevent further infections.
Ear Mite Treatment and Control
For the treatment of ear mite (Otodectes cynotis) infestations, REVOLUTION PLUS should be administered once as a single topical dose. Do not administer into the ear canal. Monthly use of REVOLUTION PLUS will control any subsequent ear mite infestations. Cleansing of the infested ears is recommended to remove debris.
Nematode Treatment and Control
For the treatment of roundworm (Toxocara cati) and intestinal hookworm (Ancylostoma tubaeforme) infections, REVOLUTION PLUS should be applied once as a single topical dose. Monthly use of REVOLUTION PLUS will control any subsequent roundworm and intestinal hookworm infections.
- CONTRAINDICATIONS:
-
WARNINGS:
Human warnings:
Not for human use. Keep this and all drugs out of the reach of children.
Do not come into contact with or allow children to contact the application site until 4 hours post application.
In humans, REVOLUTION PLUS may be irritating to skin and eyes. REVOLUTION PLUS and selamectin topical solution contain isopropyl alcohol and the preservative butylated hydroxytoluene (BHT). Reactions such as hives, itching and skin redness have been reported in humans after accidental dermal contact with selamectin topical solution. Individuals with known hypersensitivity to selamectin topical solution should use caution or consult a health care professional before applying this product on a cat. Wash hands after use and wash off any product in contact with the skin immediately with soap and water.
If contact with eyes occurs, then flush eyes copiously with water; if wearing contact lenses, rinse the eyes first then remove contact lenses and continue to rinse for 5 – 10 minutes and seek medical attention. In case of ingestion by a human, contact a physician immediately. The safety data sheet (SDS) provides more detailed occupational safety information. For a copy of the SDS or to report a suspected adverse reaction, call Zoetis at 1-888-963-8471.
Flammable - Keep away from heat, sparks, open flames or other sources of ignition. -
PRECAUTIONS:
Sarolaner, one of the ingredients in REVOLUTION PLUS, is a member of the isoxazoline class. This class has been associated with neurologic adverse reactions including tremors, ataxia, and seizures. Neurologic adverse reactions have been reported in cats receiving isoxazoline class drugs, even in cats without a history of neurologic disorders. Use with caution in cats with a history of neurologic disorders.
The safe use of REVOLUTION PLUS has not been evaluated in kittens less than 8 weeks of age. The safe use of REVOLUTION PLUS has not been evaluated in breeding, pregnant, or lactating cats.
-
ADVERSE REACTIONS:
In a field safety and effectiveness study, REVOLUTION PLUS was administered to cats with fleas. The study included a total of 430 cats (282 treated with REVOLUTION PLUS and 148 treated with imidacloprid + moxidectin once monthly for three treatments). Over the 90-day study period, all observations of potential adverse reactions were recorded. Reactions reported in the REVOLUTION PLUS group included those presented in the following table.
Adverse Reactions by Treatment GroupAdverse Reaction REVOLUTION PLUS
(n = 282)Imidacloprid + moxidectin
(n =148)Lethargy 12 (4.3%) 1 (0.7%) Skin lesions* 10 (3.5%) 3 (2.0%) Anorexia 9 (3.2%) 3 (2.0%) Pruritus 7 (2.5%) 3 (2.0%) Conjunctivitis 7 (2.5%) 1 (0.7%) Sneezing 6 (2.1%) 1 (0.7%) Administration site hair changes (alopecia) 5 (1.8%) 0 (0.0%) Administration site lesions (scabbing) 2 (0.7%) 0 (0.0%) *Lesions not associated with application site.
In a second field safety and effectiveness study, REVOLUTION PLUS was administered to 124 cats with ear mites. Adverse reactions in cats treated with REVOLUTION PLUS included emesis, dermatitis and eczema, and pruritus.In a third field safety and effectiveness study, REVOLUTION PLUS was administered to 70 cats with hookworms. Adverse reactions in cats treated with REVOLUTION PLUS included diarrhea, anorexia, emesis, and lethargy.
Post-Approval Experience (2022)
The following adverse events are based on post-approval adverse drug experience reporting forREVOLUTION PLUS. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the exposure using these data.
The following adverse events reported in cats are listed in decreasing order of reporting frequency:
Application site reactions (including alopecia, lesions, erythema, and pruritus), lethargy, anorexia, vomiting, generalized pruritus, behavioral disorders (including hiding, hyperactivity, and vocalization), ataxia, muscle tremor, diarrhea, generalized alopecia, and seizure.
Contact Information
To report adverse reactions call Zoetis Inc. at 1-888-963-8471. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
-
CLINICAL PHARMACOLOGY:
Following topical administration of REVOLUTION PLUS both selamectin and sarolaner are well absorbed with mean absolute bioavailability of 40.5% and 57.9%, respectively, and distribute systemically. In cats, selamectin and sarolaner are low clearance compounds with a long half-life of 12.5 days and 41.5 days following topical administration, respectively. Following IV dose, the total body clearance for selamectin was 10.2 mL/kg/hr and for sarolaner was 3.41 mL/kg/hr. The volume of distribution at steady state was 1.26 L/kg for selamectin and 3.87 L/kg for sarolaner. The primary route of elimination for both drugs is hepatobiliary excretion (approximately 50% of dose excreted in feces).
Following repeated monthly doses of REVOLUTION PLUS, there was a less than dose proportional increase in the Cmax and AUC of sarolaner and selamectin. The accumulation of both sarolaner and selamectin was observed with Cmax plateauing after the 6th dose for sarolaner and the 3rd dose for selamectin.
-
MODE OF ACTION:
REVOLUTION PLUS contains two active pharmaceutical ingredients, selamectin and sarolaner. Selamectin is a semi-synthetic compound of the avermectin class. Selamectin paralyzes and/or kills a wide range of invertebrate parasites through interference with their chloride channel conductance causing disruption of normal neurotransmission. This inhibits the electrical activity of nerve cells in nematodes and muscle cells in arthropods leading to their paralysis and/or death. Sarolaner is an acaricide and insecticide belonging to the isoxazoline family. The primary target of action of sarolaner in insects and acarines is functional blockade of ligand-gated chloride channels (GABA-receptors and glutamate-receptors). Sarolaner blocks GABA- and glutamate-gated chloride channels in the nervous system of insects and acarines. Disruption of these receptors by sarolaner prevents the uptake of chloride ions by GABA and glutamate ion channels, thus resulting in increased nerve stimulation and death of the target parasite. Sarolaner exhibits higher functional potency to block insect/acarine receptors compared to mammalian receptors.
-
EFFECTIVENESS:
Flea Treatment and Prevention
In a well-controlled laboratory study, REVOLUTION PLUS started to kill fleas within 12 hours of treatment and killed >98% of fleas within 24 hours. During subsequent weekly infestations, REVOLUTION PLUS started to kill fleas within 6 hours and killed ≥93.8% of fleas within 12 hours through Day 28.
In a 90-day multi-center field study in the United States, conducted in households with existing flea infestations, the effectiveness of REVOLUTION PLUS against fleas was 97.2%, 99.5%, and 99.8% when assessed on Days 30, 60, and 90, respectively. Cats with signs of flea allergy dermatitis showed improvement in alopecia, dermatitis/pyodermatitis, pruritus, erythema, and papules, and scaling, as a direct result of eliminating fleas.
In two additional well-controlled laboratory studies, there was a 100% reduction in adult flea count against an existing infestation and weekly re-infestation for at least 35 days. In one of these studies in which flea egg laying was examined, no flea eggs were recovered from cats treated with REVOLUTION PLUS through Day 22, and on Day 36. Four flea eggs were recovered from one cat on Day 29, but none of these four eggs hatched and developed into adult fleas. Fleas on control cats continued to produce eggs throughout the study, with counts ranging from 110 - 1,256 eggs per cat.
Collectively, the data from the four studies (three laboratory and one field) demonstrate that REVOLUTION PLUS kills fleas before they can lay eggs, thus preventing further flea infestations from developing once treatment of the existing flea infestation has been initiated.Tapeworm Prevention
In two well-controlled laboratory studies, a single topical dose of REVOLUTION PLUS was ≥ 97.1% effective in preventing Dipylidium caninum infections in cats after multiple infestations with Dipylidium caninum-infected C. felis fleas for one month.
Tick Treatment and ControlIn well-controlled laboratory studies, REVOLUTION PLUS began to kill Ixodes scapularis ticks within 24 hours of administration and demonstrated ≥93.3% effectiveness 48 hours post re-infestation for a month. REVOLUTION PLUS demonstrated ≥91.6% effectiveness against Amblyomma maculatum 48 hours post-infestation for a month. At 72 hours post-infestation, REVOLUTION PLUS demonstrated ≥93.5% effectiveness against Dermacentor variabilis and ≥92.5% effectiveness against Amblyomma americanum for a month.
Heartworm PreventionIn two well-controlled laboratory studies, a single topical dose of REVOLUTION PLUS was 100% effective in preventing the development of heartworms in cats inoculated with infective larvae of Dirofilaria immitis 30 days prior to treatment.
Ear Mite Treatment and ControlIn a well-controlled laboratory study, a single topical dose of REVOLUTION PLUS resulted in > 99% effectiveness against an existing Otodectes cynotis infestation at 30 days post treatment. In a multi-center field study conducted in households with existing Otodectes cynotis infestations, 94.4% of cats in the group treated with REVOLUTION PLUS were negative for ear mites at Day 30.
Nematode Treatment and ControlElimination of intestinal hookworm (Ancylostoma tubaeforme) and roundworm (Toxocara cati) infections was demonstrated in well-controlled laboratory studies.
In a 60-day multi-center field study, the effectiveness of REVOLUTION PLUS in reducing Ancylostoma tubaeforme fecal egg counts was 99.4% and 99.7% on Days 30 and 60, respectively.
-
ANIMAL SAFETY:
Margin of Safety Studies: One exploratory and two pivotal margin of safety studies were conducted with REVOLUTION PLUS. In the first study, REVOLUTION PLUS was applied topically to kittens eight weeks of age at doses of 12/2 (selamectin/sarolaner) mg/kg (1X), 36/6 mg/kg (3X), 45/7.5 mg/kg (3.75X), and 60/10 mg/kg (5X) every 28 days for eight consecutive doses. One female cat in the 3.75X group was found dead on study day 115. The cause was determined to be hemorrhage in multiple tissues secondary to a low platelet count. The role of the drug in contributing to this event is undetermined. No significant changes related to REVOLUTION PLUS were observed among the remaining cats for physical examination, body weight, clinical pathology (hematology, coagulation, and serum chemistry), gross pathology, histopathology or organ weights.
In the second study, REVOLUTION PLUS was applied topically to cats 9 months of age at doses of 1X, 3X, and 5X every 28 days for six consecutive doses. Cosmetic changes at the application site occurred sporadically in all treatment groups and included wet appearance and dried white material. Hair loss at the dose site was also noted in two cats in the 1X group and one cat in the 5X group within 1-8 days after the fourth dose administered on day 84. No significant changes related to REVOLUTION PLUS were observed for physical examination, body weight, clinical pathology (hematology, coagulation, and serum chemistry).
During an exploratory margin of safety study, one cat in the 60 mg/kg/ 10 mg/kg (selamectin/sarolaner) group (5X dose group) experienced piloerection, tremors, and mydriasis approximately 24 hours after receiving the third monthly dose of the combination. Signs resolved without treatment within 2 hours. This cat completed the study, receiving 3 subsequent 5X doses with no abnormal observations.
Oral safety study: The safety of REVOLUTION PLUS administered orally to kittens was tested in case of accidental oral ingestion. Oral administration of the highest recommended topical dose of REVOLUTION PLUS to kittens resulted in transient lower food consumption and clinical findings of emesis, soft feces, and salivation. In one male, mild tremor was observed and resolved within 3 hours after dosing; the same cat demonstrated reduced activity approximately 6 hours after dosing.
Heartworm Positive Cat Safety of Selamectin: Selamectin is the active ingredient in REVOLUTION PLUS that prevents heartworm disease in cats; it has been shown that the addition of sarolaner does not interfere with this activity. In a safety study in which selamectin topical solution was applied at 4 times the recommended dose to patent heartworm infected cats, no adverse reactions were observed.
Field safety: In three well-controlled field studies, REVOLUTION PLUS was used concurrently with other medications, such as vaccines, cestocidal anthelmintics, antibacterials, sedatives, anesthetics, opioid analgesics, corticosteroids, and non-steroidal anti-inflammatories. No adverse reactions were associated with the concurrent use of REVOLUTION PLUS and other medications.
- STORAGE CONDITIONS:
-
HOW SUPPLIED:
Available in three separate dose strengths for cats of different weights (see DOSAGE AND ADMINISTRATION). REVOLUTION PLUS is available in cartons containing one, three, or six single dose tubes. The amount of liquid in tube varies for each weight range (2.8 - 5.5 lbs, 5.6 - 11 lbs, 11.1- 22 lbs).
Tubes are never completely filled. - SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 0.25 3-pack Carton
- PRINCIPAL DISPLAY PANEL - 0.5 3-pack Carton
- PRINCIPAL DISPLAY PANEL - 1 mL 3-pack Carton
-
INGREDIENTS AND APPEARANCE
REVOLUTION PLUS
selamectin and sarolaner solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-0411 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SAROLANER (UNII: DM113FTW7F) (SAROLANER - UNII:DM113FTW7F) SAROLANER 10 mg in 1 mL SELAMECTIN (UNII: A2669OWX9N) (SELAMECTIN - UNII:A2669OWX9N) SELAMECTIN 60 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-0411-1 3 in 1 CARTON 1 0.25 mL in 1 TUBE 2 NDC:54771-0411-2 6 in 1 CARTON 2 0.25 mL in 1 TUBE 3 NDC:54771-0411-3 1 in 1 CARTON 3 0.25 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141502 11/29/2018 REVOLUTION PLUS
selamectin and sarolaner solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-0412 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SAROLANER (UNII: DM113FTW7F) (SAROLANER - UNII:DM113FTW7F) SAROLANER 10 mg in 1 mL SELAMECTIN (UNII: A2669OWX9N) (SELAMECTIN - UNII:A2669OWX9N) SELAMECTIN 60 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-0412-3 1 in 1 CARTON 1 .5 mL in 1 TUBE 2 NDC:54771-0412-1 3 in 1 CARTON 2 0.5 mL in 1 TUBE 3 NDC:54771-0412-2 6 in 1 CARTON 3 0.5 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141502 11/29/2018 REVOLUTION PLUS
selamectin and sarolaner solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-0416 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELAMECTIN (UNII: A2669OWX9N) (SELAMECTIN - UNII:A2669OWX9N) SELAMECTIN 60 mg in 1 mL SAROLANER (UNII: DM113FTW7F) (SAROLANER - UNII:DM113FTW7F) SAROLANER 10 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-0416-1 3 in 1 CARTON 1 1 mL in 1 TUBE 2 NDC:54771-0416-2 6 in 1 CARTON 2 1 mL in 1 TUBE 3 NDC:54771-0416-3 1 in 1 CARTON 3 1 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141502 11/29/2018 Labeler - Zoetis Inc. (828851555)






