Label: NITROFURANTOIN (MONOHYDRATE/MACROCRYSTALS)- nitrofurantoin monohydrate/macrocrystals capsule
- NDC Code(s): 60687-633-01, 60687-633-11, 60687-633-65
- Packager: American Health Packaging
- This is a repackaged label.
- Source NDC Code(s): 65162-478
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 26, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of Nitrofurantoin capsules, USP (monohydrate/macrocrystals) and other antibacterial drugs, Nitrofurantoin ...
-
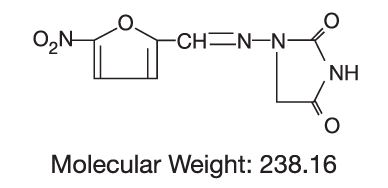
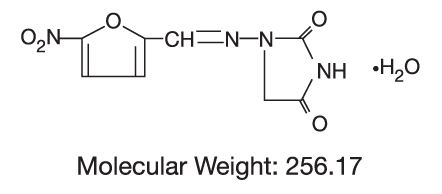
DESCRIPTIONNitrofurantoin, USP is an antibacterial agent specific for urinary tract infections. Nitrofurantoin capsules, USP (monohydrate/macrocrystals) are a hard gelatin capsule shell containing the ...
-
CLINICAL PHARMACOLOGYEach nitrofurantoin capsule (monohydrate/macrocrystals) contains two forms of nitrofurantoin. Twenty-five percent is macrocrystalline nitrofurantoin, which has slower dissolution and absorption ...
-
MICROBIOLOGYNitrofurantoin is a nitrofuran antimicrobial agent with activity against certain Gram-positive and Gram-negative bacteria. Mechanism of Action - The mechanism of the antimicrobial action of ...
-
INDICATIONS AND USAGENitrofurantoin capsules, USP (monohydrate/macrocrystals) are indicated only for the treatment of acute uncomplicated urinary tract infections (acute cystitis) caused by susceptible strains of ...
-
CONTRAINDICATIONSAnuria, oliguria, or significant impairment of renal function (creatinine clearance under 60 mL per minute or clinically significant elevated serum creatinine) are contraindications. Treatment of ...
-
WARNINGSPulmonary reactions: ACUTE, SUBACUTE, OR CHRONIC PULMONARY REACTIONS HAVE BEEN OBSERVED IN PATIENTS TREATED WITH NITROFURANTOIN. IF THESE REACTIONS OCCUR, NITROFURANTOIN SHOULD BE DISCONTINUED ...
-
PRECAUTIONSInformation for Patients - Patients should be advised to take nitrofurantoin with food (ideally breakfast and dinner) to further enhance tolerance and improve drug absorption. Patients should be ...
-
ADVERSE REACTIONSIn clinical trials of nitrofurantoin, the most frequent clinical adverse events that were reported as possibly or probably drug-related were nausea (8%), headache (6%) and flatulence (1.5%) ...
-
OVERDOSAGEOccasional incidents of acute overdosage of nitrofurantoin have not resulted in any specific symptoms other than vomiting. Induction of emesis is recommended. There is no specific antidote, but a ...
-
DOSAGE AND ADMINISTRATIONNitrofurantoin capsules (monohydrate/macrocrystals) should be taken with food. Adults and Pediatric Patients Over 12 Years: One 100 mg capsule every 12 hours for seven days.
-
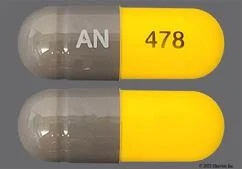
HOW SUPPLIEDNitrofurantoin capsules, USP (monohydrate/macrocrystals), 100 mg, are supplied as a gray opaque cap and yellow opaque body imprinted axially “AN” in white ink on the cap and “478” in black ink ...
-
CLINICAL STUDIESControlled clinical trials comparing nitrofurantoin 100 mg p.o. q12h and nitrofurantoin macrocrystals 50 mg p.o. q6h in the treatment of acute uncomplicated urinary tract infections demonstrated ...
-
PACKAGING INFORMATIONAmerican Health Packaging unit dose blisters (see - How Supplied section) contain drug product from Amneal Pharmaceuticals LLC as follows: (100 mg / 50 UD) NDC 60687-633-65 packaged from NDC ...
-
Package/Label Display Panel – Carton – 100 mg, 50 UDNDC 60687- 633-65 - Nitrofurantoin - Capsules, USP - (monohydrate/macrocrystals) 100 mg - 50 Capsules (5 x 10) Rx Only - URINARY TRACT ANTIBACTERIAL - Each Capsule ...
-
Package/Label Display Panel – Carton – 100 mg, 100 UDNDC 60687- 633-01 - Nitrofurantoin - Capsules, USP - (monohydrate/macrocrystals) 100 mg - 100 Capsules (10 x 10) Rx Only - URINARY TRACT ANTIBACTERIAL - Each Capsule ...
-
Package/Label Display Panel - Blister - 100 mgNitrofurantoin - Capsules, USP (monohydrate/ macrocrystals) 100 mg
-
INGREDIENTS AND APPEARANCEProduct Information