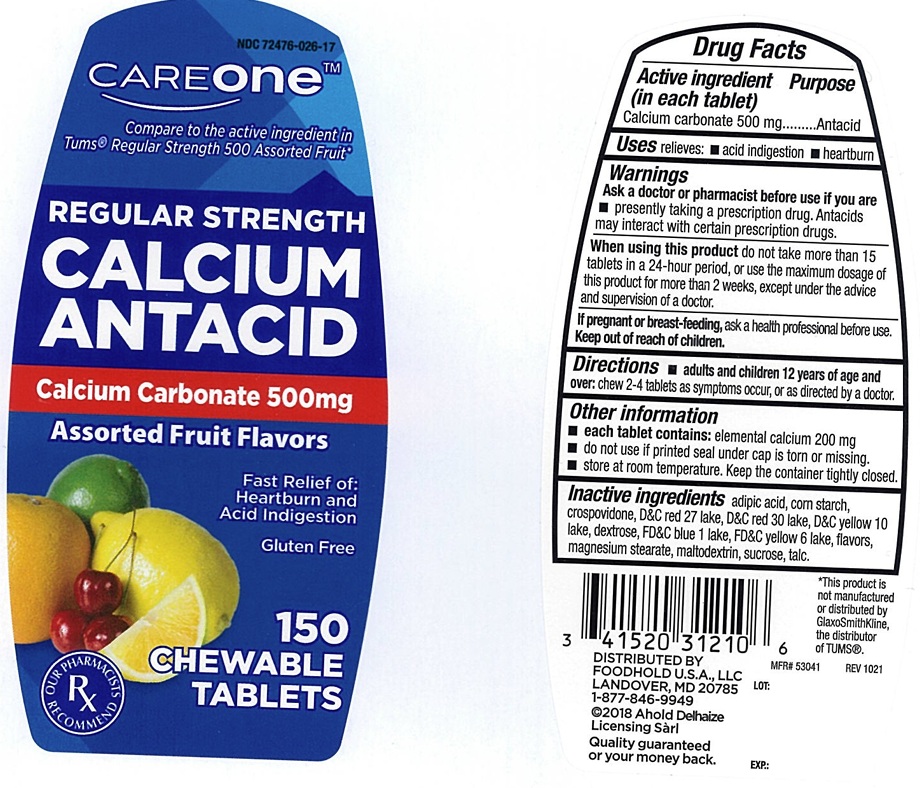
Label: CAREONE REGULAR STRENGTH CALCIUM ANTACID- calcium carbonate tablet, chewable
- NDC Code(s): 72476-026-17
- Packager: Retail Business Services, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT (in each tablet)
- PURPOSE
- USE(S)
- WARNINGS
- ASK A DOCTOR OR PHARMACIST BEFORE USE IF YOU ARE
- WHEN USING THIS PRODUCT
- IF PREGNANT OR/BREASTFEEDING,
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CAREONE REGULAR STRENGTH CALCIUM ANTACID
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72476-026 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 500 mg Inactive Ingredients Ingredient Name Strength ADIPIC ACID (UNII: 76A0JE0FKJ) STARCH, CORN (UNII: O8232NY3SJ) CROSPOVIDONE (UNII: 2S7830E561) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 30 (UNII: 2S42T2808B) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color ORANGE, YELLOW, GREEN, PINK (reddish pink) Score no score Shape ROUND Size 16mm Flavor CHERRY, LEMON, LIME, ORANGE Imprint Code G126 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72476-026-17 150 in 1 BOTTLE; Type 0: Not a Combination Product 10/13/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 10/13/2021 Labeler - Retail Business Services, LLC. (967989935) Establishment Name Address ID/FEI Business Operations Guardian Drug Company 119210276 MANUFACTURE(72476-026)