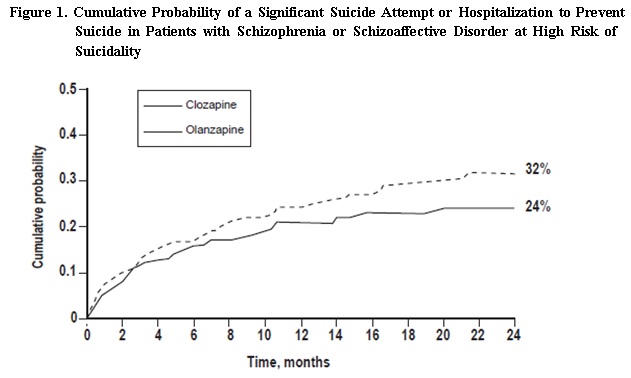
5.1 Severe Neutropenia
-
Background
-
Clozapine orally disintegrating tablets can cause neutropenia (a low absolute neutrophil count [ANC]), defined as a reduction below pre-treatment normal ...
5.1 Severe Neutropenia
Background
Clozapine orally disintegrating tablets can cause neutropenia (a low absolute neutrophil count [ANC]), defined as a reduction below pre-treatment normal levels of blood neutrophils. The ANC is usually available as a component of the complete blood count (CBC), including differential, and is more relevant to drug-induced neutropenia than is the white blood cell (WBC) count. The ANC may also be calculated using the following formula: ANC equals the Total WBC count multiplied by the total percentage of neutrophils obtained from the differential (neutrophil “segs” plus neutrophil “bands”). Other granulocytes (basophils and eosinophils) contribute minimally to neutropenia and their measurement is not necessary [see Adverse Reactions (6.2)]. Neutropenia may be mild, moderate, or severe (see Tables 2 and 3). To improve and standardize understanding, “severe neutropenia” replaces the previous terms severe leukopenia, severe granulocytopenia, or agranulocytosis.
Severe neutropenia, ANC less than (<) 500/µL, occurs in a small percentage of patients taking clozapine orally disintegrating tablets and is associated with an increase in the risk of serious and potentially fatal infections. Risk of neutropenia appears greatest during the first 18 weeks on treatment and then declines. The mechanism by which clozapine orally disintegrating tablets causes neutropenia is unknown and is not dose-dependent.
Two separate management algorithms are provided below, the first for patients in the general population, and the second for patients identified to have baseline neutropenia.
Clozapine Orally Disintegrating Tablets Treatment and Monitoring in the General Patient Population (see Table 2)
Obtain a CBC, including the ANC value, prior to initiating treatment with clozapine orally disintegrating tablets to ensure the presence of a normal baseline neutrophil count (equal to or greater than 1500/µL) and to permit later comparisons. Patients in the general population with an ANC equal to or greater than (≥)1500/µL are considered within normal range (Table 2) and are eligible to initiate treatment. Weekly ANC monitoring is required for all patients during the first 6 months of treatment. If a patient’s ANC remains equal to or greater than 1500/µL for the first 6 months of treatment, monitoring frequency may be reduced to every 2 weeks for the next 6 months. If the ANC remains equal to or greater than 1500/µL for the second 6 months of continuous therapy, ANC monitoring frequency may be reduced to once every 4 weeks thereafter.
Clozapine Orally Disintegrating Tablets Treatment and Monitoring in Patients with Benign Ethnic Neutropenia (see Table 3)
Benign ethnic neutropenia (BEN) is a condition observed in certain ethnic groups whose average ANC values are lower than “standard” laboratory ranges for neutrophils. It is most commonly observed in individuals of African descent (approximate prevalence of 25 to 50%), some Middle Eastern ethnic groups, and in other non-Caucasian ethnic groups with darker skin. BEN is more common in men. Patients with BEN have normal hematopoietic stem-cell number and myeloid maturation, are healthy, and do not suffer from repeated or severe infections. They are not at increased risk for developing clozapine orally disintegrating tablets-induced neutropenia. Additional evaluation may be needed to determine if baseline neutropenia is due to BEN. Consider hematology consultation before initiating or during clozapine orally disintegrating tablets treatment as necessary.
Patients with BEN require a different ANC algorithm for clozapine orally disintegrating tablets management due to their lower baseline ANC levels. Table 3 provides guidelines for managing clozapine orally disintegrating tablets treatment and ANC monitoring in patients with BEN.
General Guidelines for Management of All Patients with Fever or with Neutropenia
- Fever: Interrupt clozapine orally disintegrating tablets as a precautionary measure in any patient who develops fever, defined as a temperature of 38.5°C [101.3°F] or greater, and obtain an ANC level. Fever is often the first sign of neutropenic infection.
- ANC less than 1000/µL: If fever occurs in any patient with an ANC less than 1000/µL, initiate appropriate workup and treatment for infection and refer to Tables 2 or 3 for management.
- Consider hematology consultation.
- See Neuroleptic Malignant Syndrome (NMS) and Fever under WARNINGS AND PRECAUTIONS (5) and Instructions for Patients, under PATIENT COUNSELING INFORMATION (17).
Rechallenge after an ANC less than 500/µL (severe neutropenia)
For some patients who experience severe clozapine orally disintegrating tablets-related neutropenia, the risk of serious psychiatric illness from discontinuing clozapine orally disintegrating tablets treatment may be greater than the risk of rechallenge (e.g., patients with severe schizophrenic illness who have no treatment options other than clozapine orally disintegrating tablets). A hematology consultation may be useful in deciding to rechallenge a patient. In general, however, do not rechallenge patients who develop severe neutropenia with clozapine orally disintegrating tablets or a clozapine product.
If a patient will be rechallenged, the clinician should consider thresholds provided in Tables 2 and 3, the patient’s medical and psychiatric history, a discussion with the patient and his/her caregiver about the benefits and risks of clozapine orally disintegrating tablets rechallenge, and the severity and characteristics of the neutropenic episode.
Using Clozapine Orally Disintegrating Tablets with Other Drugs Associated with Neutropenia
It is unclear if concurrent use of other drugs known to cause neutropenia increases the risk or severity of clozapine orally disintegrating tablets-induced neutropenia. There is no strong scientific rationale to avoid clozapine orally disintegrating tablets treatment in patients concurrently treated with these drugs. If clozapine orally disintegrating tablets are used concurrently with an agent known to cause neutropenia (e.g., some chemotherapeutic agents), consider monitoring patients more closely than the treatment guidelines provided in Tables 2 and 3. Consult with the treating oncologist in patients receiving concomitant chemotherapy.
5.2 Clozapine REMS Program
Clozapine orally disintegrating tablets are only available through a restricted program under a REMS called the Clozapine REMS Program because of the risk of severe neutropenia.
Notable requirements of the Clozapine REMS Program include:
- Healthcare professionals who prescribe clozapine orally disintegrating tablets must be certified with the program by enrolling and completing training.
- Patients who receive clozapine orally disintegrating tablets must be enrolled in the program and comply with the ANC testing and monitoring requirements.
- Pharmacies dispensing clozapine orally disintegrating tablets must be certified with the program by enrolling and completing training and must only dispense to patients who are eligible to receive clozapine orally disintegrating tablets.
For more information call Aurobindo Pharma USA, Inc. at 1-866-850-2876.
5.3 Orthostatic Hypotension, Bradycardia, and Syncope
Hypotension, bradycardia, syncope, and cardiac arrest have occurred with clozapine treatment. The risk is highest during the initial titration period, particularly with rapid dose-escalation.
These reactions can occur with the first dose, at doses as low as 12.5 mg. These reactions can be fatal. The syndrome is consistent with neurally mediated reflex bradycardia (NMRB).
Treatment must begin at a maximum dose of 12.5 mg once daily or twice daily. The total daily dose can be increased in increments of 25 mg to 50 mg per day, if well-tolerated, to a target dose of 300 mg to 450 mg per day (administered in divided doses) by the end of 2 weeks. Subsequently, the dose can be increased weekly or twice weekly, in increments of up to 100 mg. The maximum dose is 900 mg per day. Use cautious titration and a divided dosage schedule to minimize the risk of serious cardiovascular reactions [see Dosage and Administration (2.3)]. Consider reducing the dose if hypotension occurs. When restarting clozapine orally disintegrating tablets in patients who have had even a brief interruption in treatment with clozapine orally disintegrating tablets, the dosage must be reduced. This is necessary to minimize the risk of hypotension, bradycardia, and syncope [see Dosage and Administration (2.6)].
Use clozapine orally disintegrating tablets cautiously in patients with cardiovascular disease (history of myocardial infarction or ischemia, heart failure, or conduction abnormalities), cerebrovascular disease, and conditions which would predispose patients to hypotension (e.g., concomitant use of antihypertensives, dehydration and hypovolemia).
5.4 Falls
Clozapine orally disintegrating tablets may cause somnolence, postural hypotension, and motor and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic treatment.
5.5 Seizures
Seizure has been estimated to occur in association with clozapine use at a cumulative incidence at one year of approximately 5%, based on the occurrence of one or more seizures in 61 of 1743 patients exposed to clozapine during its clinical testing prior to domestic marketing (i.e., a crude rate of 3.5%). The risk of seizure is dose-related. Initiate treatment with a low dose (12.5 mg), titrate slowly, and use divided dosing.
Use caution when administering clozapine orally disintegrating tablets to patients with a history of seizures or other predisposing risk factors for seizure (e.g., head trauma or other CNS pathology, use of medications that lower the seizure threshold, or alcohol abuse). Because of the substantial risk of seizure associated with clozapine orally disintegrating tablets use, caution patients about engaging in any activity where sudden loss of consciousness could cause serious risk to themselves or others (e.g., driving an automobile, operating complex machinery, swimming, climbing).
5.6 Myocarditis, Pericarditis, Cardiomyopathy, and Mitral Valve Incompetence
Myocarditis, pericarditis, and cardiomyopathy have occurred with the use of clozapine orally disintegrating tablets. These reactions can be fatal. Discontinue clozapine orally disintegrating tablets and obtain a cardiac evaluation upon suspicion of myocarditis, pericarditis, or cardiomyopathy. Generally, patients with a history of clozapine-associated myocarditis, pericarditis, or cardiomyopathy should not be rechallenged with clozapine orally disintegrating tablets. However, if the benefit of clozapine orally disintegrating tablets treatment is judged to outweigh the potential risks of recurrence, the clinician may consider rechallenge with clozapine orally disintegrating tablets in consultation with a cardiologist.
Consider the possibility of myocarditis or cardiomyopathy in patients receiving clozapine orally disintegrating tablets who present with chest pain, dyspnea, persistent tachycardia at rest, palpitations, fever, flu-like symptoms, hypotension, other signs or symptoms of heart failure, or electrocardiographic findings (low voltages, ST-T abnormalities, arrhythmias, right axis deviation, and poor R wave progression). Myocarditis and pericarditis most frequently present within the first 2 months of clozapine treatment. Symptoms of cardiomyopathy generally occur later than clozapine-associated myocarditis and usually after 8 weeks of treatment. However, myocarditis, pericarditis, and cardiomyopathy can occur at any period during treatment with clozapine orally disintegrating tablets. In patients who are diagnosed with cardiomyopathy while taking clozapine orally disintegrating tablets mitral valve incompetence has been reported.
5.7 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality in this population. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Clozapine orally disintegrating tablets are not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning].
5.8 Gastrointestinal Hypomotility with Severe Complications
Severe gastrointestinal adverse reactions have occurred with the use of clozapine orally disintegrating tablets, primarily due to its potent anticholinergic effects and resulting gastrointestinal hypomotility. In post marketing experience, reported effects range from constipation to paralytic ileus. Increased frequency of constipation and delayed diagnosis and treatment increased the risk of severe complications of gastrointestinal hypomotility, which can result in fecal impaction, megacolon, and intestinal obstruction, ischemia, infarction, perforation, ulceration, or necrosis [see Adverse Reaction (6.2)]. These reactions have resulted in hospitalization, surgery, and death. The risk for severe adverse reactions is further increased with anticholinergic medications (and other medications that decrease gastrointestinal peristalsis); therefore, concomitant use should be avoided when possible [see Warnings and Precautions (5.16), Drug Interactions (7.1)].
Prior to initiating clozapine orally disintegrating tablets, screen for constipation and treat as necessary. Subjective symptoms of constipation may not accurately reflect the degree of gastrointestinal hypomotility in clozapine orally disintegrating tablets treated patients. Therefore, reassess bowel function frequently with careful attention to any changes in the frequency or character of bowel movements, as well as signs and symptoms of complications of hypomotility (e.g., nausea, vomiting, abdominal distension, abdominal pain). If constipation or gastrointestinal hypomotility are identified, monitor closely and treat promptly with appropriate laxatives, as necessary, to prevent severe complications. Consider prophylactic laxatives in high risk patients.
5.9 Eosinophilia
Eosinophilia, defined as a blood eosinophil count of greater than 700/µL, has occurred with clozapine treatment. In clinical trials, approximately 1% of patients developed eosinophilia. Clozapine-related eosinophilia usually occurs during the first month of treatment. In some patients, it has been associated with myocarditis, pancreatitis, hepatitis, colitis, and nephritis. Such organ involvement could be consistent with a drug reaction with eosinophilia and systemic symptoms syndrome (DRESS), also known as drug induced hypersensitivity syndrome (DIHS). If eosinophilia develops during clozapine orally disintegrating tablets treatment, evaluate promptly for signs and symptoms of systemic reactions, such as rash or other allergic symptoms, myocarditis, or other organ-specific disease associated with eosinophilia. If clozapine-related systemic disease is suspected, discontinue clozapine orally disintegrating tablets immediately.
If a cause of eosinophilia unrelated to clozapine is identified (e.g., asthma, allergies, collagen vascular disease, parasitic infections, and specific neoplasms), treat the underlying cause and continue clozapine orally disintegrating tablets.
Clozapine-related eosinophilia has also occurred in the absence of organ involvement and can resolve without intervention. There are reports of successful rechallenge after discontinuation of clozapine, without recurrence of eosinophilia. In the absence of organ involvement, continue clozapine orally disintegrating tablets under careful monitoring. If the total eosinophil count continues to increase over several weeks in the absence of systemic disease, the decision to interrupt clozapine orally disintegrating tablets therapy and rechallenge after the eosinophil count decreases should be based on the overall clinical assessment, in consultation with an internist or hematologist.
5.10 QT Interval Prolongation
QT prolongation, Torsades de Pointes and other life-threatening ventricular arrhythmias, cardiac arrest, and sudden death have occurred with clozapine treatment. When prescribing clozapine orally disintegrating tablets, consider the presence of additional risk factors for QT prolongation and serious cardiovascular reactions. Conditions that increase these risks include the following: history of QT prolongation, long QT syndrome, family history of long QT syndrome or sudden cardiac death, significant cardiac arrhythmia, recent myocardial infarction, uncompensated heart failure, treatment with other medications that cause QT prolongation, treatment with medications that inhibit the metabolism of clozapine orally disintegrating tablets, and electrolyte abnormalities.
Prior to initiating treatment with clozapine orally disintegrating tablets, perform a careful physical examination, medical history, and concomitant medication history. Consider obtaining a baseline ECG and serum chemistry panel. Correct electrolyte abnormalities. Discontinue clozapine orally disintegrating tablets if the QTc interval exceeds 500 msec. If patients experience symptoms consistent with Torsades de Pointes or other arrhythmias (e.g., syncope, presyncope, dizziness, or palpitations), obtain a cardiac evaluation and discontinue clozapine orally disintegrating tablets.
Use caution when administering concomitant medications that prolong the QT interval or inhibit the metabolism of clozapine orally disintegrating tablets. Drugs that cause QT prolongation include: specific antipsychotics (e.g., ziprasidone, iloperidone, chlorpromazine, thioridazine, mesoridazine, droperidol, pimozide), specific antibiotics (e.g., erythromycin, gatifloxacin, moxifloxacin, sparfloxacin), Class 1A antiarrhythmic medications (e.g., quinidine, procainamide) or Class III antiarrhythmics (e.g., amiodarone, sotalol), and others (e.g., pentamidine, levomethadyl acetate, methadone, halofantrine, mefloquine, dolasetron mesylate, probucol or tacrolimus). Clozapine orally disintegrating tablets are primarily metabolized by CYP isoenzymes 1A2, 2D6, and 3A4. Concomitant treatment with inhibitors of these enzymes can increase the concentration of clozapine orally disintegrating tablets [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Hypokalemia and hypomagnesemia increase the risk of QT prolongation. Hypokalemia can result from diuretic therapy, diarrhea, and other causes. Use caution when treating patients at risk for significant electrolyte disturbance, particularly hypokalemia. Obtain baseline measurements of serum potassium and magnesium levels, and periodically monitor electrolytes. Correct electrolyte abnormalities before initiating treatment with clozapine orally disintegrating tablets.
5.11 Metabolic Changes
Atypical antipsychotic drugs, including clozapine orally disintegrating tablets, have been associated with metabolic changes that can increase cardiovascular and cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and body weight gain. While atypical antipsychotic drugs may produce some metabolic changes, each drug in the class has its own specific risk profile.
Hyperglycemia and Diabetes Mellitus
Hyperglycemia, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, has been reported in patients treated with atypical antipsychotics including clozapine orally disintegrating tablets. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse reactions is not completely understood. However, epidemiological studies suggest an increased risk of treatment-emergent, hyperglycemia-related adverse reactions in patients treated with the atypical antipsychotics. Precise risk estimates for hyperglycemia-related adverse reactions in patients treated with atypical antipsychotics are not available.
Patients with an established diagnosis of diabetes mellitus who are started on clozapine orally disintegrating tablets should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients required continuation of anti-diabetic treatment despite discontinuation of the suspect drug.
In a pooled data analysis of 8 studies in adult subjects with schizophrenia, the mean changes in fasting glucose concentration in the clozapine and chlorpromazine groups were +11 mg/dL and +4 mg/dL respectively. A higher proportion of the clozapine group demonstrated categorical increases from baseline in fasting glucose concentrations, compared to the chlorpromazine group (Table 4). The clozapine doses were 100 to 900 mg per day (mean modal dose: 512 mg per day). The maximum chlorpromazine dose was 1800 mg per day (mean modal dose: 1029 mg per day). The median duration of exposure was 42 days for clozapine and chlorpromazine.
Dyslipidemia
Undesirable alterations in lipids have occurred in patients treated with atypical antipsychotics, including clozapine orally disintegrating tablets. Clinical monitoring, including baseline and periodic follow-up lipid evaluations in patients using clozapine orally disintegrating tablets, is recommended.
In a pooled data analysis of 10 studies in adult subjects with schizophrenia, clozapine treatment was associated with increases in serum total cholesterol. No data were collected on LDL and HDL cholesterol. The mean increase in total cholesterol was 13 mg/dL in the clozapine group and 15 mg/dL in the chlorpromazine group. In a pooled data analysis of 2 studies in adult subjects with schizophrenia, clozapine treatment was associated with increases in fasting serum triglyceride. The mean increase in fasting triglyceride was 71 mg/dL (54%) in the clozapine group and 39 mg/dL (35%) in the chlorpromazine group (Table 5). In addition, clozapine treatment was associated with categorical increases in serum total cholesterol and triglyceride, as illustrated in Table 6. The proportion of patients with categorical increases in total cholesterol or fasting triglyceride increased with the duration of exposure. The median duration of clozapine and chlorpromazine exposure was 45 days and 38 days, respectively. The clozapine dose range was 100 mg to 900 mg daily; the maximum chlorpromazine dose was 1800 mg daily.
Weight Gain
Weight gain has occurred with the use of antipsychotics, including clozapine orally disintegrating tablets. Monitor weight during treatment with clozapine orally disintegrating tablets. Table 7 summarizes the data on weight gain by the duration of exposure pooled from 11 studies with clozapine and active comparators. The median duration of exposure was 609, 728, and 42 days, in the clozapine, olanzapine, and chlorpromazine group, respectively.
Table 8 summarizes pooled data from 11 studies in adult subjects with schizophrenia demonstrating weight gain ≥7% of body weight relative to baseline. The median duration of exposure was 609, 728, and 42 days, in the clozapine, olanzapine, and chlorpromazine group, respectively.
5.12 Neuroleptic Malignant Syndrome
Antipsychotic drugs including clozapine orally disintegrating tablets can cause a potentially fatal symptom complex referred to as Neuroleptic Malignant Syndrome (NMS). Clinical manifestations of NMS include hyperpyrexia, muscle rigidity, altered mental status, and autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias). Associated findings can include elevated creatine phosphokinase (CPK), myoglobinuria, rhabdomyolysis, and acute renal failure.
The diagnostic evaluation of patients with this syndrome is complicated. It is important to consider the presence of other serious medical conditions (e.g., severe neutropenia, infection, heat stroke, primary CNS pathology, central anticholinergic toxicity, extrapyramidal symptoms, and drug fever).
The management of NMS should include (1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, (2) intensive symptomatic treatment and medical monitoring, and (3) treatment of co-morbid medical conditions. There is no general agreement about specific pharmacological treatments for NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. NMS can recur. Monitor closely if restarting treatment with antipsychotics.
NMS has occurred with clozapine monotherapy and with concomitant CNS-active medications, including lithium.
5.13 Hepatotoxicity
Severe, life threatening, and in some cases fatal hepatotoxicity including hepatic failure, hepatic necrosis, and hepatitis have been reported in patients treated with clozapine [see Adverse Reactions (6.2)]. Monitor for the appearance of signs and symptoms of hepatotoxicity such as fatigue, malaise, anorexia, nausea, jaundice, bilirubinemia, coagulopathy, and hepatic encephalopathy. Perform serum tests for liver injury and consider permanently discontinuing treatment if hepatitis or transaminase elevations combined with other systemic symptoms are due to clozapine.
5.14 Fever
During clozapine therapy, patients have experienced transient, clozapine-related fever. The peak incidence is within the first 3 weeks of treatment. While this fever is generally benign and self- limited, it may necessitate discontinuing treatment. The fever can be associated with an increase or decrease in WBC count. Carefully evaluate patients with fever to rule out severe neutropenia or infection. Consider the possibility of NMS [see Warnings and Precautions (5.11)].
5.15 Pulmonary Embolism
Pulmonary embolism and deep vein thrombosis have occurred in patients treated with clozapine. Consider the possibility of pulmonary embolism in patients who present with deep vein thrombosis, acute dyspnea, chest pain, or with other respiratory signs and symptoms. Whether pulmonary embolus and deep vein thrombosis can be attributed to clozapine or some characteristic(s) of patients is not clear.
5.16 Anticholinergic Toxicity
Clozapine orally disintegrating tablets has potent anticholinergic effects. Treatment with clozapine orally disintegrating tablets can result in CNS and peripheral anticholinergic toxicity, especially at higher dosages, or in overdose situations [see Overdosage (10)]. Use with caution in patients with a current diagnosis or prior history of constipation, urinary retention, clinically significant prostatic hypertrophy, or other conditions in which anticholinergic effects can lead to significant adverse reactions. When possible, avoid concomitant use with other anticholinergic medications because the risk for anticholinergic toxicity or severe gastrointestinal adverse reactions is increased [see Warnings and Precautions (5.8), Drug Interactions (7.1)].
5.17 Interference with Cognitive and Motor Performance
Clozapine orally disintegrating tablets can cause sedation and impairment of cognitive and motor performance. Caution patients about operating hazardous machinery, including automobiles, until they are reasonably certain that clozapine orally disintegrating tablets does not affect them adversely. These reactions may be dose-related. Consider reducing the dose if they occur.
5.18 Tardive Dyskinesia
Tardive dyskinesia (TD) has occurred in patients treated with antipsychotic drugs, including clozapine orally disintegrating tablets. The syndrome consists of potentially irreversible, involuntary, dyskinetic movements. The risk of TD and the likelihood that it will become irreversible are believed to increase with greater durations of treatment and higher total cumulative doses. However, the syndrome can develop after relatively brief treatment periods at low doses. Prescribe clozapine orally disintegrating tablets in a manner that is most likely to minimize the risk of developing TD. Use the lowest effective dose and the shortest duration necessary to control symptoms. Periodically assess the need for continued treatment. Consider discontinuing treatment if TD occurs. However, some patients may require treatment with clozapine orally disintegrating tablets despite the presence of the syndrome.
There is no known treatment for TD. However, the syndrome may remit partially or completely if treatment is discontinued. Antipsychotic treatment, itself, may suppress (or partially suppress) the signs and symptoms, and it has the potential to mask the underlying process. The effect of symptom suppression on the long-term course of TD is unknown.
5.19 Patients with Phenylketonuria
Phenylketonuric patients should be informed that clozapine orally disintegrating tablets contains phenylalanine (a component of aspartame). Each 12.5 mg, orally disintegrating tablet contains 0.70 mg phenylalanine. Each 25 mg, orally disintegrating tablet contains 1.40 mg phenylalanine. Each 100 mg, orally disintegrating tablet contains 5.61 mg phenylalanine. Each 150 mg, orally disintegrating tablet contains 8.42 mg phenylalanine. Each 200 mg, orally disintegrating tablet contains 11.23 mg phenylalanine.
5.20 Cerebrovascular Adverse Reactions
In controlled trials, elderly patients with dementia-related psychosis treated with some atypical antipsychotics had an increased risk (compared to placebo) of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack), including fatalities. The mechanism for this increased risk is not known. An increased risk cannot be excluded for clozapine orally disintegrating tablets or other antipsychotics or other patient populations. Clozapine orally disintegrating tablets should be used with caution in patients with risk factors for cerebrovascular adverse reactions.
5.21 Recurrence of Psychosis and Cholinergic Rebound after Abrupt Discontinuation of Clozapine Orally Disintegrating Tablets
If abrupt discontinuation of clozapine orally disintegrating tablets are necessary (because of severe neutropenia or another medical condition, for example) [see Dosage and Administration (2.4), Warnings and Precautions (5.1)], monitor carefully for the recurrence of psychotic symptoms and adverse reactions related to cholinergic rebound, such as profuse sweating, headache, nausea, vomiting, and diarrhea.
Close