Label: MORPHINE SULFATE injection, solution, concentrate
- NDC Code(s): 0409-1134-03, 0409-1134-05, 0409-1135-02
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 13, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFOR INTRAVENOUS USE ONLY - NOT FOR INTRATHECAL OR EPIDURAL USE - Fliptop Vial - Rx only
-
BOXED WARNING
(What is this?)Addiction, Abuse, and Misuse - Morphine Sulfate Injection exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each ...
WARNING: ADDICTION, ABUSE, AND MISUSE; LIFE-THREATENING RESPIRATORY DEPRESSION; NEONATAL OPIOID WITHDRAWAL SYNDROME; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Addiction, Abuse, and Misuse
Morphine Sulfate Injection exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient's risk prior to prescribing Morphine Sulfate Injection, and monitor all patients regularly for the development of these behaviors and conditions (see WARNINGS).
Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression may occur with use of Morphine Sulfate Injection. Monitor for respiratory depression, especially during initiation of Morphine Sulfate Injection or following a dose increase. Because of delay in maximum central nervous system (CNS) effect with intravenously administered morphine (30 min), rapid intravenous administration may result in overdosing (see WARNINGS).
Neonatal Opioid Withdrawal Syndrome
Prolonged use of Morphine Sulfate Injection during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available (see WARNINGS).
CloseRisks From Concomitant Use With Benzodiazepines Or Other CNS Depressants
Concomitant use of opioids with benzodiazepines or other CNS depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death (see WARNINGS, Drug Interactions).
- Reserve concomitant prescribing of Morphine Sulfate Injection and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate.
- Limit dosages and durations to the minimum required.
- Follow patients for signs and symptoms of respiratory depression and sedation.
-
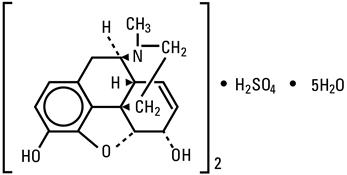
DESCRIPTIONMorphine is a tertiary nitrogen base containing a phenanthrene nucleus; it has two hydroxyl groups, one phenolic and the other alcoholic (secondary). The sulfate salt occurs as white, feathery ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Morphine is a full opioid agonist and is relatively selective for the mu-opioid receptor, although it can bind to other opioid receptors at higher doses. The principal ...
-
INDICATIONS AND USAGEMorphine sulfate is indicated for the relief of severe pain. It is used preoperatively to sedate the patient and allay apprehension, facilitate anesthesia induction and reduce anesthetic dosage ...
-
CONTRAINDICATIONSMorphine Sulfate Injection is contraindicated in patients with: Significant respiratory depression (see WARNINGS) Acute or severe bronchial asthma in an unmonitored setting or in the absence of ...
-
WARNINGSContains Sulfites - The product which contains antioxidant (25 mg/mL and 50 mg/mL concentrations – see DESCRIPTION and HOW SUPPLIED), contains sodium metabisulfite, a sulfite that may cause ...
-
PRECAUTIONSGeneral - Parenteral Therapy - Give by very slow intravenous injection, in the form of a diluted solution. Rapid intravenous injection of morphine and other narcotic analgesics increases the ...
-
ADVERSE REACTIONSThe following serious adverse reactions are described, or described in greater detail, in other sections: Addiction, Abuse, and Misuse (see WARNINGS) Life-Threatening Respiratory Depression (see ...
-
DRUG ABUSE AND DEPENDENCEControlled Substance - Morphine Sulfate Injection contains morphine, a Schedule II controlled substance. Abuse - Morphine Sulfate Injection contains morphine, a substance with a high potential ...
-
OVERDOSAGEClinical Presentation - Acute overdose with Morphine Sulfate Injection can be manifested by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration ...
-
DOSAGE AND ADMINISTRATIONTHESE PRODUCTS ARE INTENDED FOR SLOW INTRAVENOUS USE ONLY. RAPID INTRAVENOUS ADMINISTRATION MAY RESULT IN CHEST WALL RIGIDITY. NOT FOR INTRATHECAL OR EPIDURAL USE. For Relief of Pain and as ...
-
HOW SUPPLIEDMorphine Sulfate Injection, USP, is available in glass fliptop vials as follows: 25 mg/mL Morphine Sulfate Injection, USP, Preservative Free (no bacteriostat or antioxidant added). Single-dose ...
-
SPL UNCLASSIFIED SECTIONHospira, Inc., Lake Forest, IL 60045 USA - LAB-1040-4.0 - Revised September 2021
-
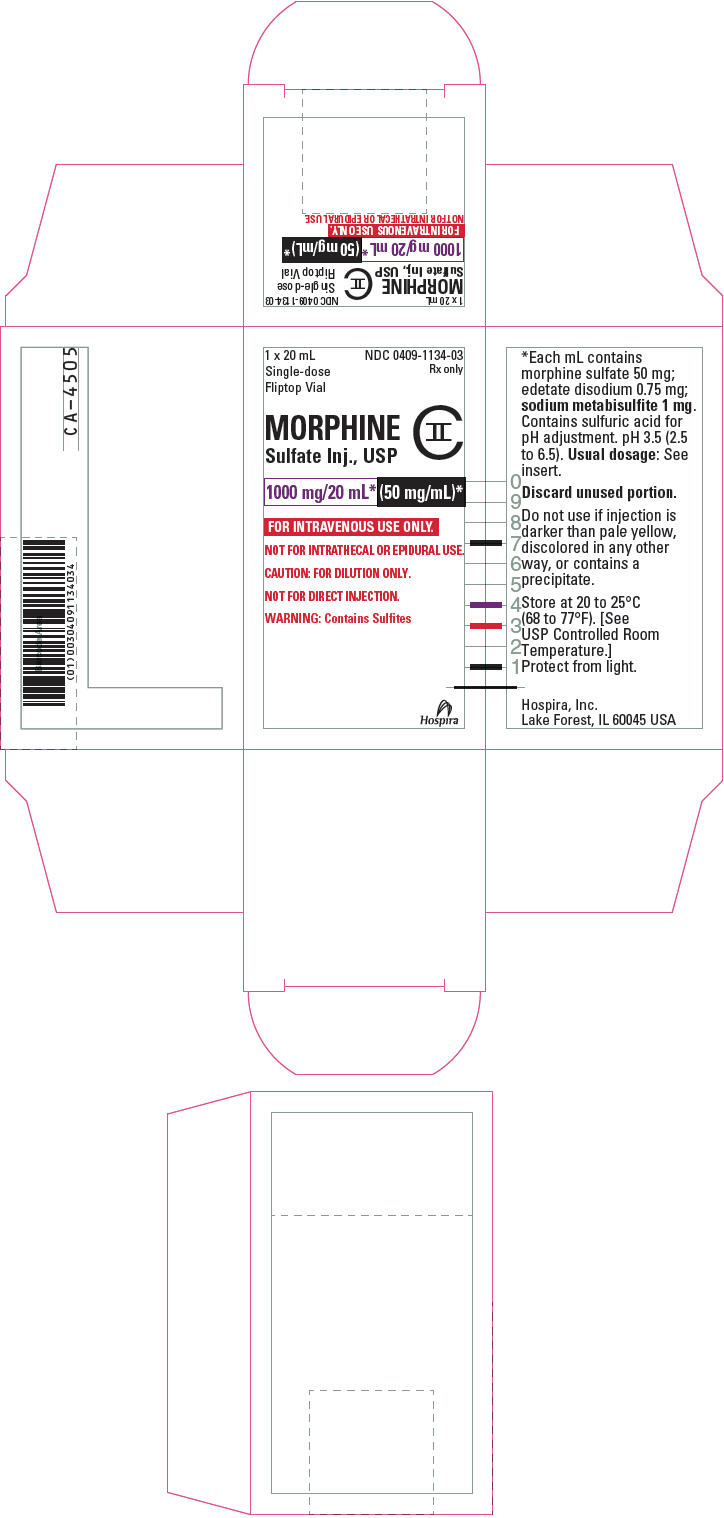
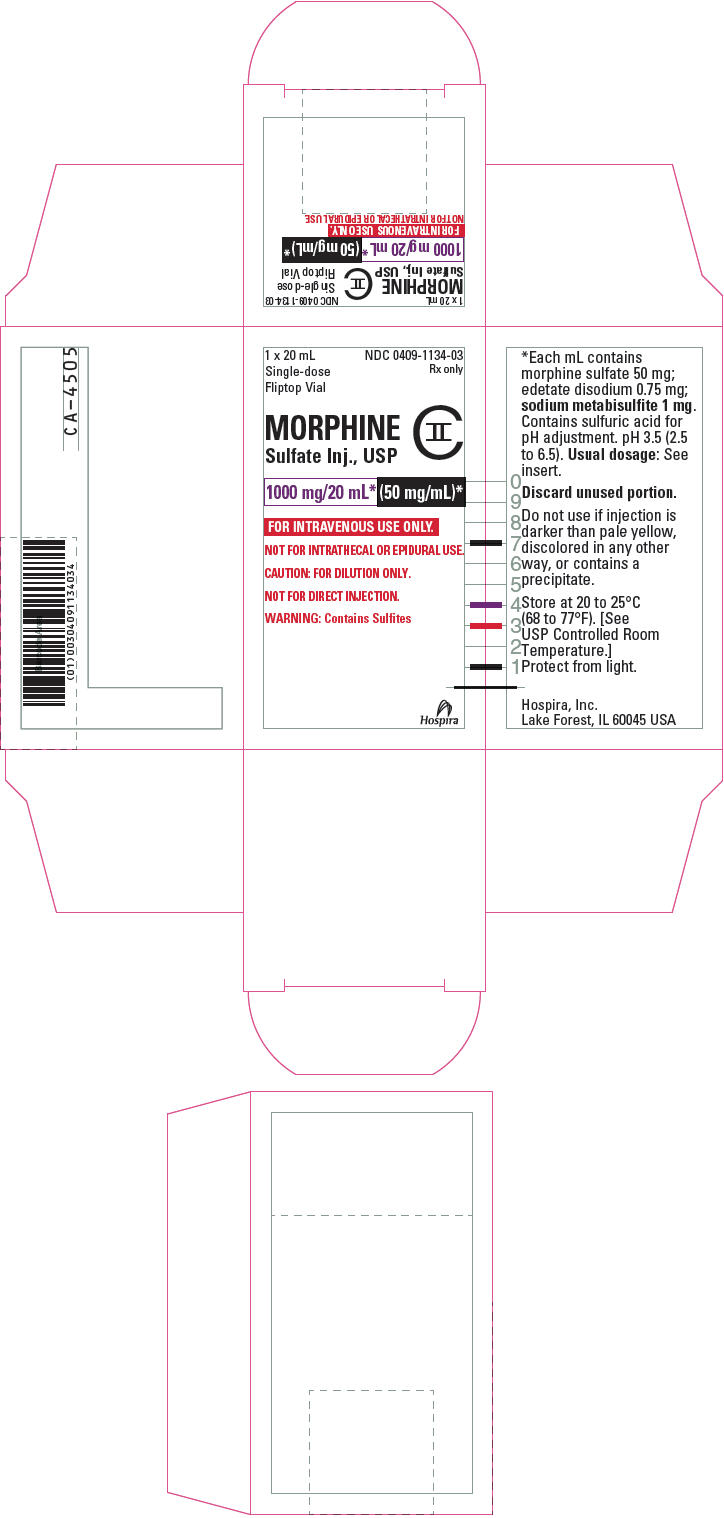
PRINCIPAL DISPLAY PANEL - 1000 mg/20 mL Vial Label20 mL - Single-dose Fliptop Vial - NDC 0409-1134-03 - Rx only - MORPHINE - Sulfate Inj., USP - CII - 1000 mg/20 mL* (50 mg/mL)* FOR INTRAVENOUS USE ONLY. NOT FOR INTRATHECAL OR EPIDURAL USE. CAUTION: FOR ...
-
PRINCIPAL DISPLAY PANEL - 1000 mg/20 mL Vial Carton1 x 20 mL - Single-dose - Fliptop Vial - NDC 0409-1134-03 - Rx only - MORPHINE - Sulfate Inj., USP - CII - 1000 mg/20 mL* (50 mg/mL)* FOR INTRAVENOUS USE ONLY. NOT FOR INTRATHECAL OR EPIDURAL ...
-
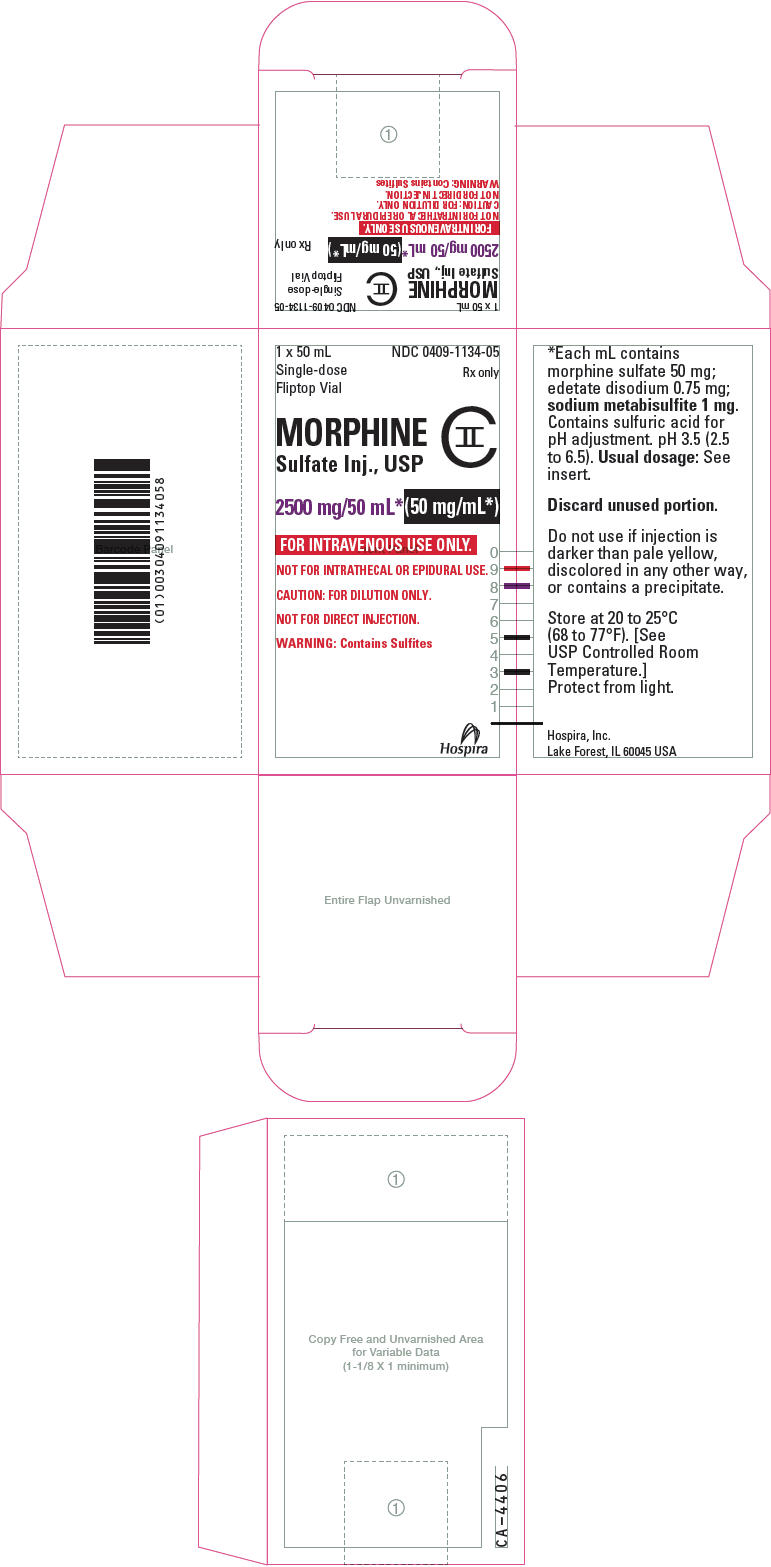
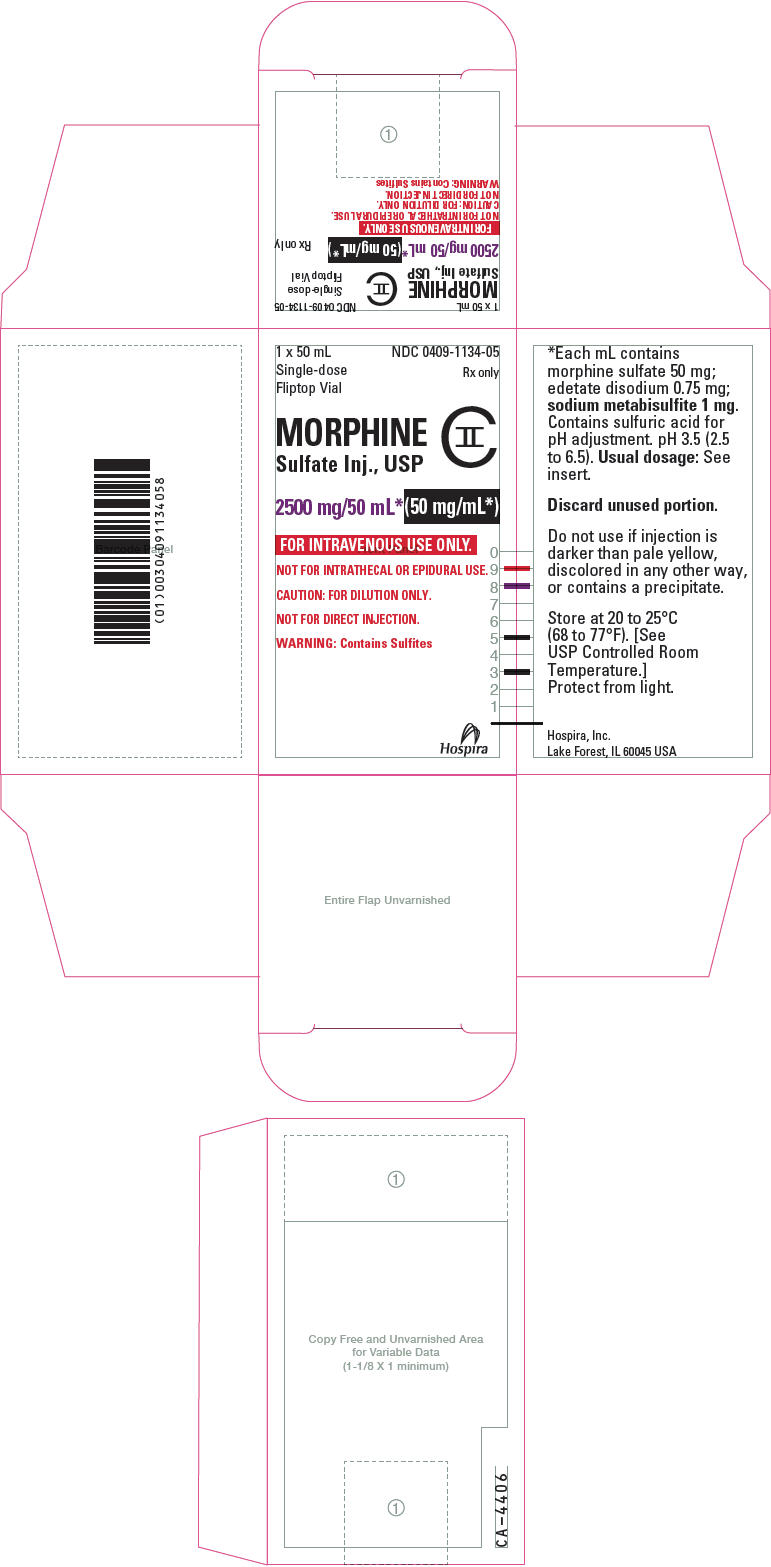
PRINCIPAL DISPLAY PANEL - 2500 mg/50 mL Vial LabelNDC 0409-1134-05 - 50 mL - Single-dose Fliptop Vial - Rx only - MORPHINE - Sulfate Inj., USP - CII - 2500 mg/50 mL* (50 mg/mL*) FOR INTRAVENOUS USE ONLY. NOT FOR INTRATHECAL OR EPIDURAL USE. CAUTION: FOR ...
-
PRINCIPAL DISPLAY PANEL - 2500 mg/20 mL Vial Carton1 x 50 mL - Single-dose - Fliptop Vial - NDC 0409-1134-05 - Rx only - MORPHINE - Sulfate Inj., USP - CII - 2500 mg/50 mL*(50 mg/mL*) FOR INTRAVENOUS USE ONLY. NOT FOR INTRATHECAL OR EPIDURAL ...
-
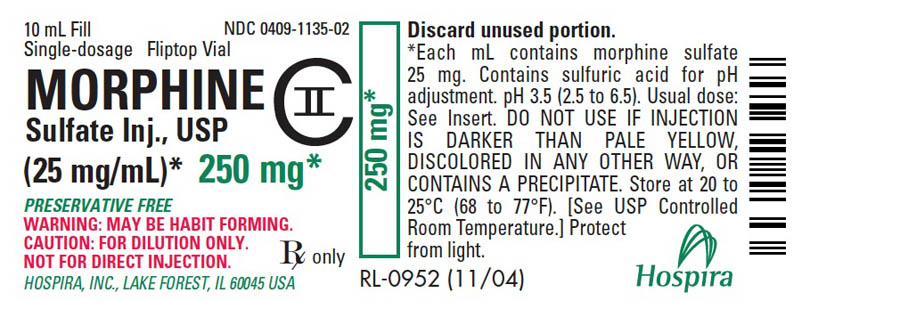
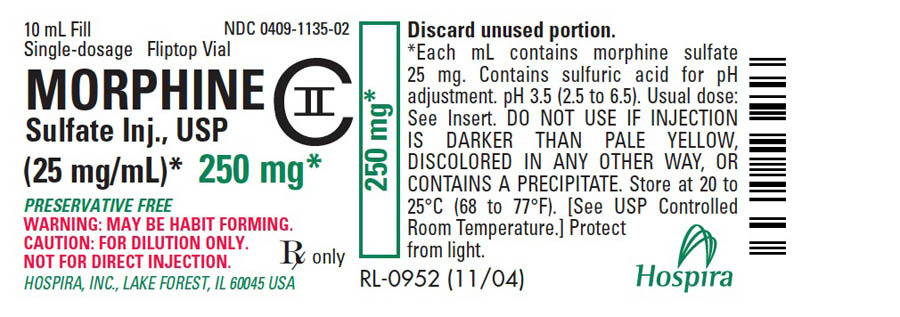
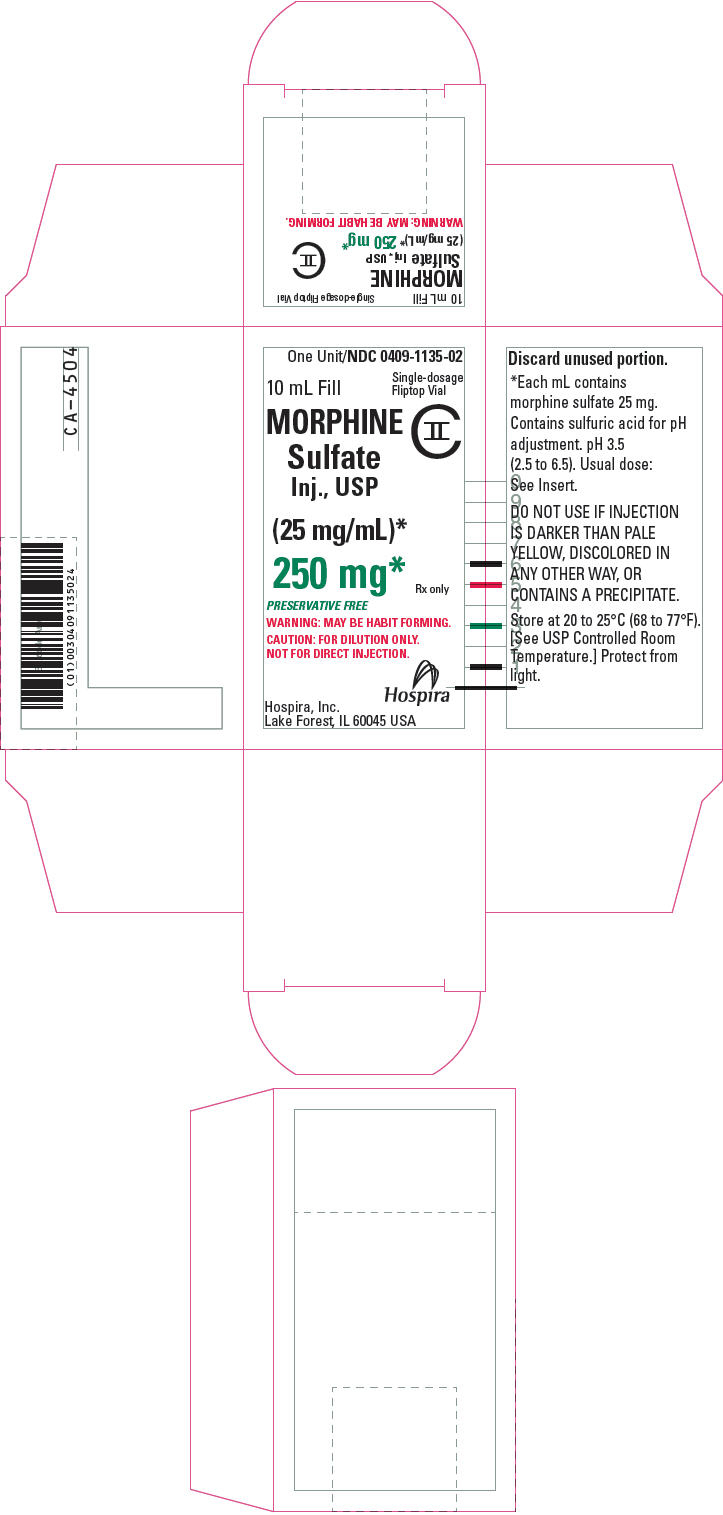
PRINCIPAL DISPLAY PANEL - 250 mg Vial Label10 mL Fill - NDC 0409-1135-02 - Single-dosage Fliptop Vial - MORPHINE - Sulfate Inj., USP - CII - (25 mg/mL)* 250 mg* PRESERVATIVE FREE - WARNING: MAY BE HABIT FORMING. CAUTION: FOR DILUTION ONLY. NOT ...
-
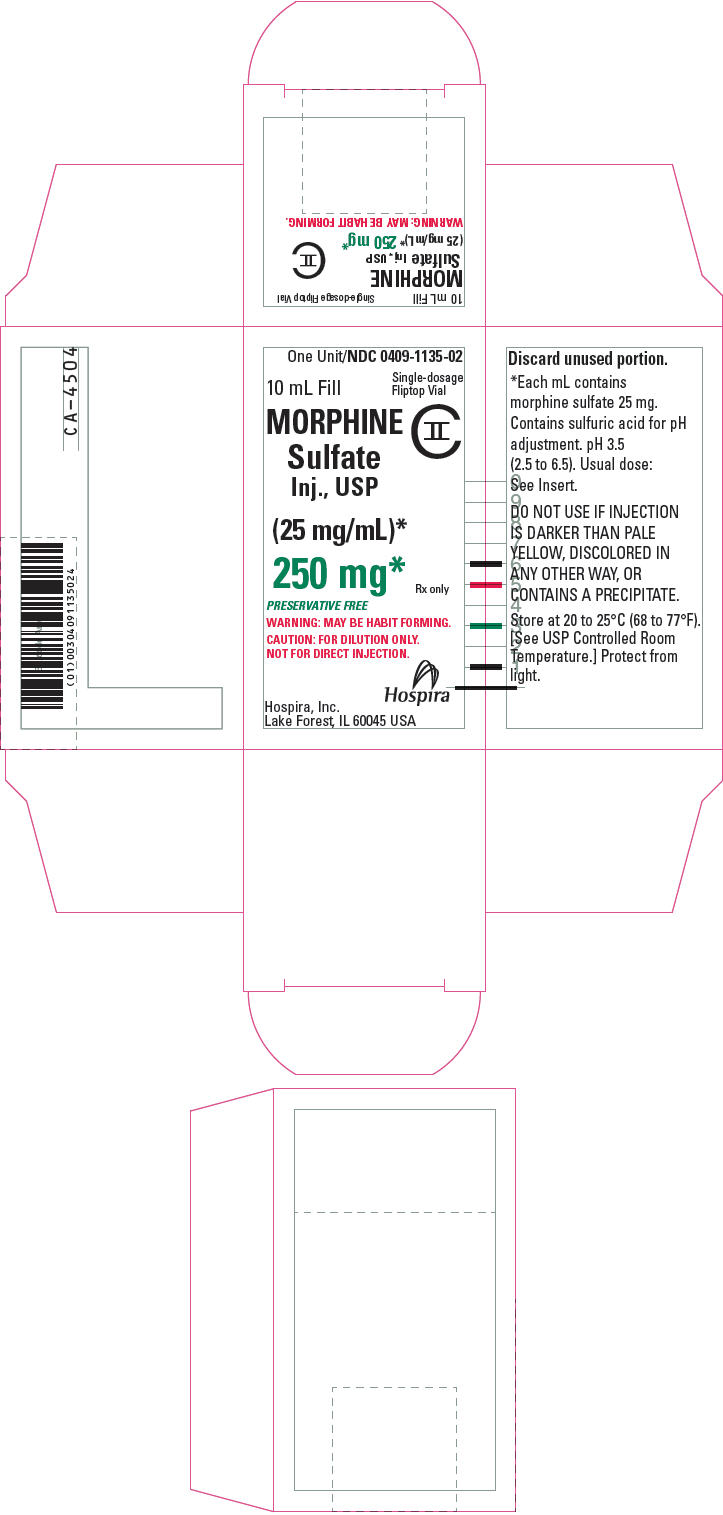
PRINCIPAL DISPLAY PANEL - 250 mg Vial CartonOne Unit/NDC 0409-1135-02 - 10 mL Fill - Single-dosage - Fliptop Vial - MORPHINE - Sulfate - Inj., USP - CII - (25 mg/mL)* 250 mg* Rx only - PRESERVATIVE FREE - WARNING: MAY BE HABIT FORMING. CAUTION: FOR ...
-
INGREDIENTS AND APPEARANCEProduct Information