Label: ZOLPIDEM TARTRATE tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 43353-617-07, 43353-617-10, 43353-617-14, 43353-617-15, view more - Packager: Aphena Pharma Solutions - Tennessee, LLC
- This is a repackaged label.
- Source NDC Code(s): 0603-6468, 0603-6469
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 10, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use zolpidem tartrate tablets safely and effectively. See full prescribing information for zolpidem tartrate tablets - Zolpidem ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZolpidem tartrate tablets are indicated for the short-term treatment of insomnia characterized by difficulties with sleep initiation. Zolpidem tartrate tablets have been shown to decrease sleep ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage in Adults - Use the lowest effective dose for the patient. The recommended initial dose is 5 mg for women and either 5 or 10 mg for men, taken only once per night immediately before ...
-
3 DOSAGE FORMS AND STRENGTHSZolpidem tartrate tablets are available in 5 mg and 10 mg strength tablets for oral administration. Tablets are not scored. Zolpidem tartrate tablets 5 mg are pink, film-coated, capsule-shaped ...
-
4 CONTRAINDICATIONSZolpidem tartrate tablets are contraindicated in patients with known hypersensitivity to zolpidem. Observed reactions include anaphylaxis and angioedema [see Warnings and Precautions ...
-
5 WARNINGS AND PRECAUTIONS5.1 CNS Depressant Effects and Next-Day Impairment - Zolpidem tartrate tablets, like other sedative-hypnotic drugs, have central nervous system (CNS) depressant effects. Co-administration with ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in greater detail in other sections of the labeling: CNS-depressant effects and next-day impairment [see Warnings and Precautions (5.1) ...
-
7 DRUG INTERACTIONS7.1 CNS-active Drugs - Co-administration of zolpidem with other CNS depressants increases the risk of CNS depression [see Warnings and Precautions (5.1)]. Zolpidem tartrate was evaluated in ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic effects: Pregnancy Category C - There are no adequate and well-controlled studies of zolpidem tartrate tablets in pregnant women. Studies in children to assess the ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - Zolpidem tartrate is classified as a Schedule IV controlled substance by federal regulation. 9.2 Abuse - Abuse and addiction are separate and distinct from physical ...
-
10 OVERDOSAGE10.1 Signs and Symptoms - In postmarketing experience of overdose with zolpidem tartrate alone, or in combination with CNS-depressant agents, impairment of consciousness ranging from somnolence ...
-
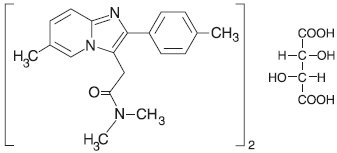
11 DESCRIPTIONZolpidem tartrate is a gamma-aminobutyric acid (GABA) A agonist of the imidazopyridine class and is available in 5 mg and 10 mg strength tablets for oral administration. Chemically, zolpidem is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Zolpidem, the active moiety of zolpidem tartrate, is a hypnotic agent with a chemical structure unrelated to benzodiazepines, barbiturates, or other drugs with known ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: Zolpidem was administered to mice and rats for 2 years at oral doses of 4, 18, and 80 mg base/kg. In mice, these ...
-
14 CLINICAL STUDIES14.1 Transient Insomnia - Normal adults experiencing transient insomnia (n = 462) during the first night in a sleep laboratory were evaluated in a double-blind, parallel group, single-night trial ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRepackaged by Aphena Pharma Solutions - TN. See Repackaging Information for available configurations. Zolpidem Tartrate Tablets are available as: 5 mg, pink, film-coated ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Medication Guide). Inform patients and their families about the benefits and risks of treatment with zolpidem tartrate tablets. Inform patients of the ...
-
MEDICATION GUIDE
CIV - ZOLPIDEM TARTRATE TABLETS - Read the Medication Guide that comes with zolpidem tartrate tablets before you start taking it and each time you get a refill. There may be new information. This ...
-
SPL UNCLASSIFIED SECTIONManufactured for: QUALITEST PHARMACEUTICALS - Huntsville, AL 35811 - Manufactured by: Vintage Pharmaceuticals - Huntsville, AL 35811 - or - Manufactured by: Patheon Inc. Whitby, Ontario ...
- SPL UNCLASSIFIED SECTION
-
Repackaging InformationPlease reference the How Supplied section listed above for a description of individual tablets or capsules. This drug product has been received by Aphena Pharma - TN in a manufacturer or ...
-
PRINCIPAL DISPLAY PANEL - 5mgNDC 43353-620 - Zolpidem Tartrate 5mg - Rx Only
-
PRINCIPAL DISPLAY PANEL - 10mgNDC 43353-617 - Zolpidem Tartrate 10mg - Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information