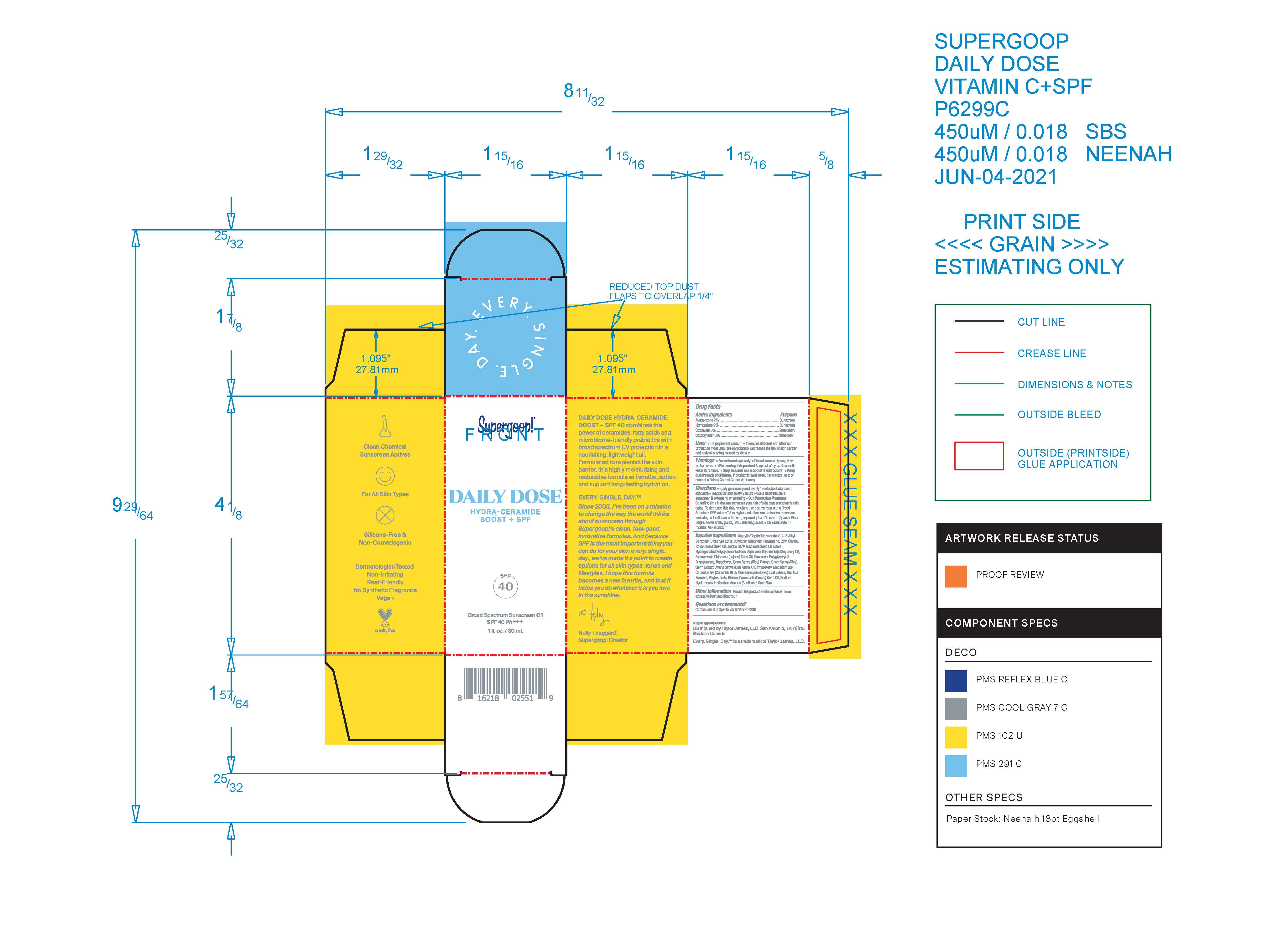
Label: DAILY DOSE HYDRA-CERAMIDE BOOST BROAD SPECTRUM SUNSCREEN SPF 40- avobenzone, homosalate, octisalate, octocrylene oil
- NDC Code(s): 75936-306-01, 75936-306-02, 75936-306-03
- Packager: Supergoop, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
- apply generously and evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- Use a water resistant Sunscreen if swimming or sweating
- Sun Protection Measures Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sun glasses
- Children under 6 months: Ask a doctor
-
INACTIVE INGREDIENT
Inactive Ingredients Caprylic/Capric Triglyceride, C12-15 Alkyl Benzoate, Dicaprylyl Ether, Butyloctyl Salicylate, Polybutene, Ethyl Olivate, Rosa Canina Seed Oil, Jojoba Oil/ Macadamia Seed Oil Esters, Hydrogenated Polycyclopentadiene, Squalane, Glycine Soja (Soybean) Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Squalene, Polyglyceryl-3 Diisostearate, Tocopherol, Oryza Sativa (Rice) Extract, Oryza Sativa (Rice) Germ Extract, Avena Sativa (Oat) Kernel Oil, Phytosteryl Macadamiate, Ceradmide NP (Ceramide III B), Olea Europaea (Olive) Leaf Extract, Bacillus Ferment, Phytosterols, Ricinus communis (Castor) Seed Oil, Sodium Hyaluronate, Helianthus Annuus (Sunflower) Seed Wax
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DAILY DOSE HYDRA-CERAMIDE BOOST BROAD SPECTRUM SUNSCREEN SPF 40
avobenzone, homosalate, octisalate, octocrylene oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-306 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TOCOPHEROL (UNII: R0ZB2556P8) JOJOBA OIL (UNII: 724GKU717M) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ETHYL OLIVATE (UNII: KKJ108Y20W) ROSA CANINA SEED OIL (UNII: MHT97MG5P8) SQUALANE (UNII: GW89575KF9) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) SOYBEAN OIL (UNII: 241ATL177A) CERAMIDE NP (UNII: 4370DF050B) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) SQUALENE (UNII: 7QWM220FJH) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DICAPRYLYL ETHER (UNII: 77JZM5516Z) CASTOR OIL (UNII: D5340Y2I9G) HYALURONATE SODIUM (UNII: YSE9PPT4TH) OAT KERNEL OIL (UNII: 3UVP41R77R) PHYTOSTERYL MACADAMIATE (UNII: 233VSF903M) RICE GERM (UNII: 7N2B70SFEZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-306-01 1.5 mL in 1 PACKET; Type 0: Not a Combination Product 08/26/2021 2 NDC:75936-306-02 5 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/26/2021 3 NDC:75936-306-03 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/26/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/26/2021 Labeler - Supergoop, LLC (117061743)