Label: FELODIPINE tablet, film coated
- NDC Code(s): 71335-2175-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 61442-432
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
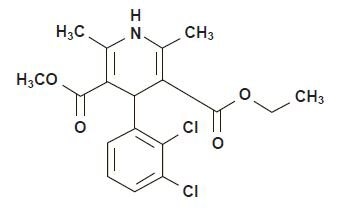
DESCRIPTIONFelodipine is a calcium antagonist (calcium channel blocker). Felodipine is a - dihydropyridine derivative that is chemically described as ± ethyl methyl ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Felodipine is a member of the dihydropyridine class of calcium channel - antagonists (calcium channel blockers). It reversibly competes with ...
-
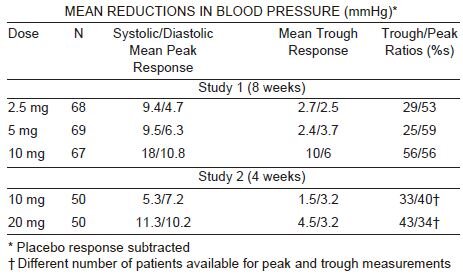
INDICATIONS AND USAGEFelodipine extended-release tablets, USP are indicated for the treatment of - hypertension, to lower blood pressure. Lowering blood pressure lowers the ...
-
CONTRAINDICATIONSFelodipine extended-release tablets are contraindicated in patients who are - hypersensitive to this product.
-
PRECAUTIONSGeneral - Hypotension - Felodipine, like other calcium antagonists, may occasionally precipitate - significant hypotension and, rarely ...
-
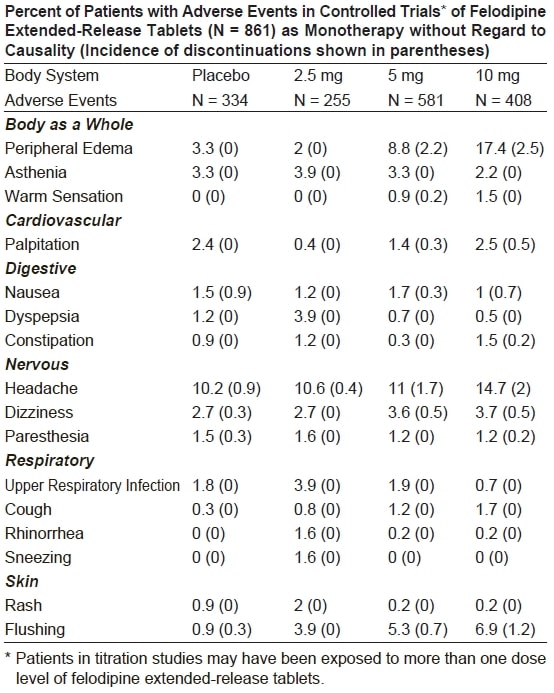
ADVERSE REACTIONSIn controlled studies in the United States and overseas, approximately 3,000 - patients were treated with felodipine as either the extended-release or the ...
-
OVERDOSAGEOral doses of 240 mg/kg and 264 mg/kg in male and female mice, respectively, and 2390 mg/kg and 2250 mg/kg in male and female rats, respectively ...
-
DOSAGE AND ADMINISTRATIONThe recommended starting dose is 5 mg once a day. Depending on the patient's - response, the dosage can be decreased to 2.5 mg or increased to 10 mg ...
-
HOW SUPPLIEDThe 5 mg tablets are orange film-coated, round, unscored tablets debossed - with “YSP 210” on one side of the tablets. NDC: 71335-2175-1: 30 Tablets in a BOTTLE - Repackaged/Relabeled by: Bryant Ranch ...
-
PRINCIPAL DISPLAY PANELFelodipine ER 5mg Tablet
-
INGREDIENTS AND APPEARANCEProduct Information