Label: ACULAR- ketorolac tromethamine solution/ drops
- NDC Code(s): 0023-2181-05
- Packager: Allergan, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ACULAR safely and effectively. See full prescribing information for ACULAR. ACULAR® (ketorolac tromethamine ...
-
Table of ContentsTable of Contents
-
1
INDICATIONS AND USAGE
ACULAR is indicated for the temporary relief of ocular itching due to seasonal allergic conjunctivitis. ACULAR is also indicated for the treatment of postoperative inflammation in patients who ...
-
2
DOSAGE AND ADMINISTRATION
2.1 - Recommended Dosage - Temporary Relief of Ocular Itching Due to Seasonal Allergic Conjunctivitis - The recommended dosage of ACULAR is one drop four times a day to the affected ...
-
3
DOSAGE FORMS AND STRENGTHS
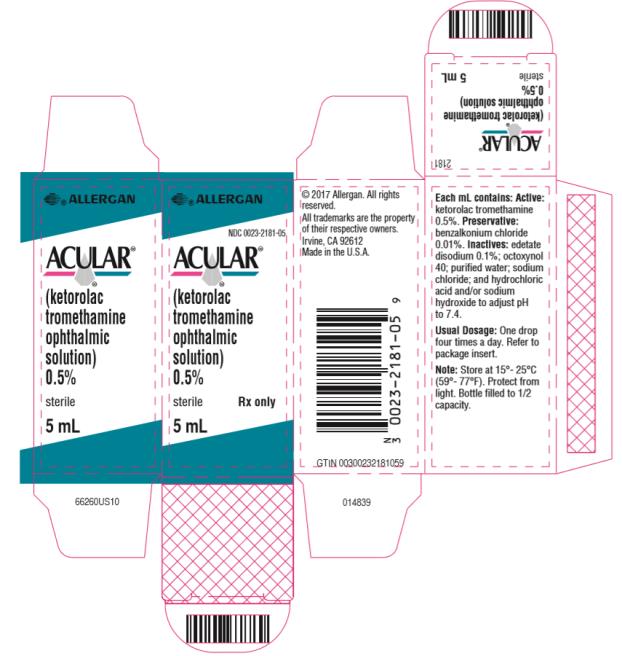
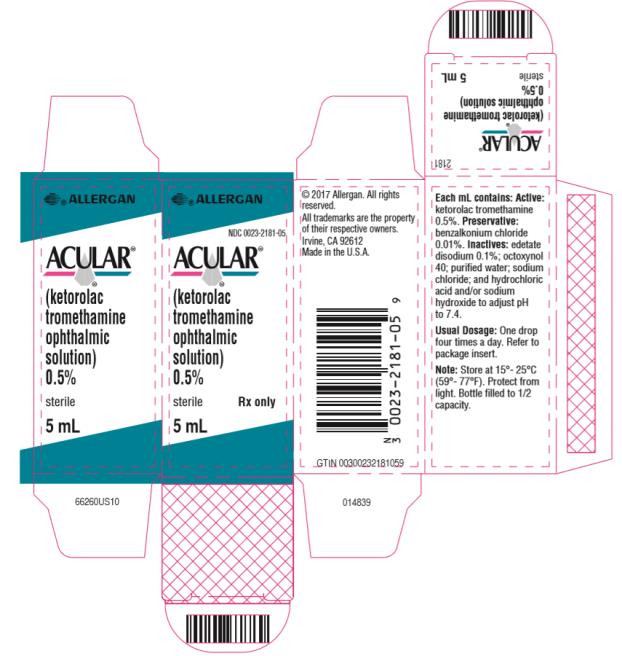
10 mL size bottle filled with 5 mL of ketorolac tromethamine ophthalmic solution, 0.5% (5 mg/mL).
-
4
CONTRAINDICATIONS
ACULAR solution is contraindicated in patients with previously demonstrated hypersensitivity to any of the ingredients in the formulation [see Adverse Reactions (6.1)].
-
5
WARNINGS AND PRECAUTIONS
5.1 - Delayed Healing - Topical nonsteroidal anti-inflammatory drugs (NSAIDs) may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use ...
-
6
ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling: Delayed Healing [see Warnings and Precautions (5.1)] Cross-Sensitivity or Hypersensitivity [see Warnings and ...
-
8
USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Risk Summary - There are no adequate and well-controlled studies with ACULAR in pregnant women. No evidence of teratogenicity has been observed in rats or rabbits with ...
-
11
DESCRIPTION
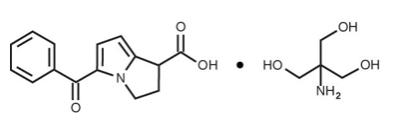
ACULAR (ketorolac tromethamine ophthalmic solution) 0.5% is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for topical ophthalmic use. Its chemical name is ...
-
12
CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - Ketorolac tromethamine is a nonsteroidal anti-inflammatory drug which, when administered systemically, has demonstrated analgesic, anti-inflammatory, and ...
-
14
CLINICAL STUDIES
Two controlled clinical studies showed that ketorolac tromethamine ophthalmic solution was significantly more effective than its vehicle in relieving ocular itching caused by seasonal allergic ...
-
16
HOW SUPPLIED/STORAGE AND HANDLING
ACULAR (ketorolac tromethamine ophthalmic solution) 0.5% is supplied sterile, in white opaque plastic (LDPE) bottles with white droppers, with gray high impact polystyrene (HIPS) caps as ...
-
17
PATIENT COUNSELING INFORMATION
Slow or Delayed Healing - Advise patients of the possibility that slow or delayed healing may occur while using nonsteroidal anti-inflammatory drugs (NSAIDs). Avoiding Contamination of the ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - abbvie - NDC 0023-2181-05 - ACULAR® (ketorolac - tromethamine - ophthalmic - solution) 0.5% sterile Rx only - 5 mL
-
INGREDIENTS AND APPEARANCEProduct Information