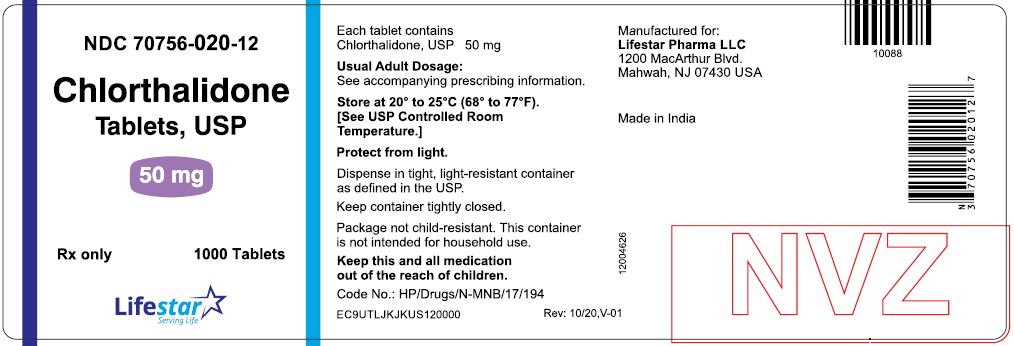
Label: CHLORTHALIDONE tablet
- NDC Code(s): 70756-011-11, 70756-011-12, 70756-020-11, 70756-020-12
- Packager: Lifestar Pharma LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Rx only
-
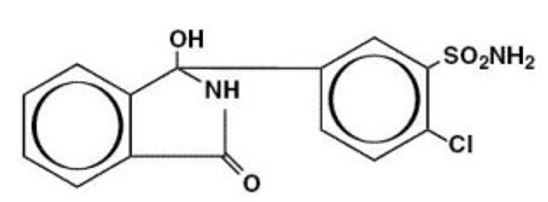
DESCRIPTIONChlorthalidone an oral antihypertensive/diuretic. It is a monosulfamyl diuretic that differs chemically from thiazide diuretics in that a double-ring system is incorporated in its structure. It ...
-
CLINICAL PHARMACOLOGYChlorthalidone is an oral diuretic with prolonged action (48-72 hours) and low toxicity. The major portion of the drug is excreted unchanged by the kidneys. The diuretic effect of the drug occurs ...
-
INDICATIONS AND USAGEDiuretics such as chlorthalidone are indicated in the management of hypertension either as the sole therapeutic agent or to enhance the effect of other antihypertensive drugs in the more severe ...
-
CONTRAINDICATIONSAnuria. Known hypersensitivity to chlorthalidone or other sulfonamide-derived drugs.
-
WARNINGSChlorthalidone should be used with caution in severe renal disease. In patients with renal disease, chlorthalidone or related drugs may precipitate azotemia. Cumulative effects of the drug may ...
-
PRECAUTIONSGeneral - Hypokalemia may develop with chlorthalidone as with any other diuretic, especially with brisk diuresis when severe cirrhosis is present or during concomitant use of corticosteroids or ...
-
ADVERSE REACTIONSThe following adverse reactions have been observed, but there is not enough systematic collection of data to support an estimate of their frequency. Gastrointestinal System Reactions: anorexia ...
-
OVERDOSAGESymptoms of acute overdosage include nausea, weakness, dizziness, and disturbances of electrolyte balance. The oral LD50 of the drug in the mouse and the rat is more than 25,000 mg/kg body ...
-
DOSAGE AND ADMINISTRATIONTherapy should be initiated with the lowest possible dose. This dose should be titrated according to individual patient response to gain maximal therapeutic benefit while maintaining lowest ...
-
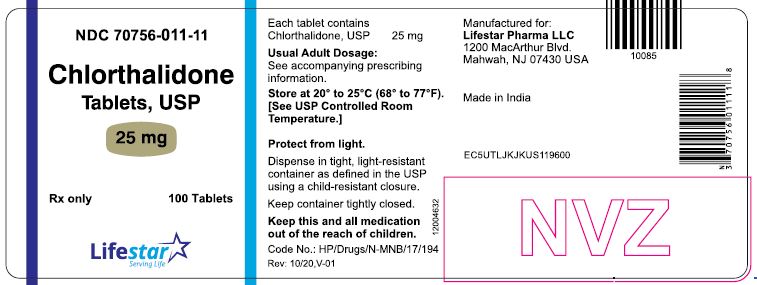
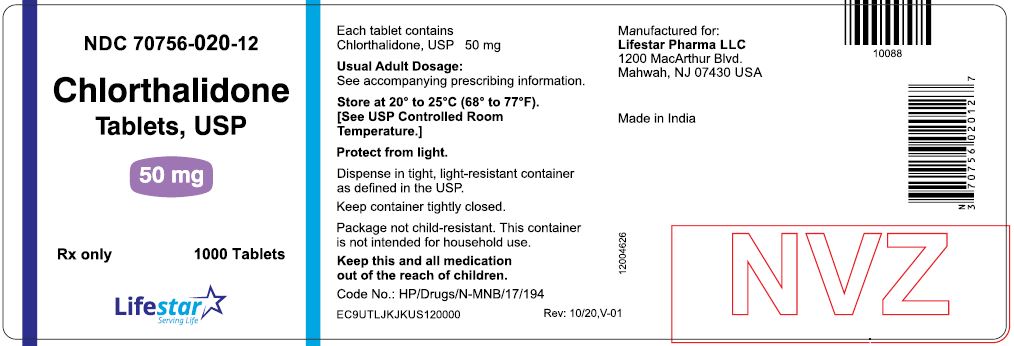
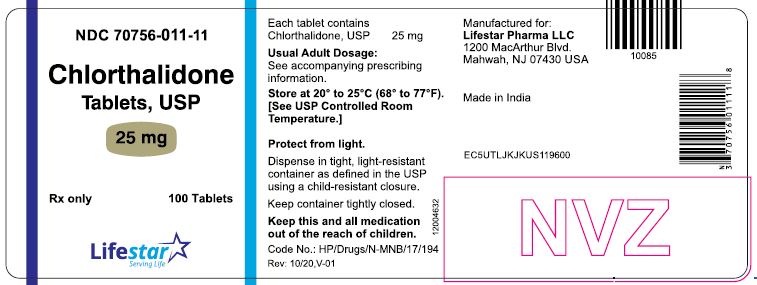
HOW SUPPLIEDChlorthalidone Tablets, USP are available containing 25 mg or 50 mg of chlorthalidone, USP. The 25 mg tablets are white to off-white, round, unscored tablets, debossed with 'L1' on one side of ...
-
ANIMAL PHARMACOLOGYBiochemical studies in animals have suggested reasons for the prolonged effect of chlorthalidone. Absorption from the gastrointestinal tract is slow due to its low solubility. After passage to ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70756-011-11 - Chlorthalidone - Tablets, USP - 25 mg - Rx only - 100 Tablets - NDC 70756-011-12 - Chlorthalidone - Tablets, USP - 25 mg - Rx only - 1000 Tablets
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70756-020-11 - Chlorthalidone - Tablets, USP - 50 mg - Rx only - 100 Tablets - NDC 70756-020-12 - Chlorthalidone - Tablets, USP - 50 mg - Rx only - 1000 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information