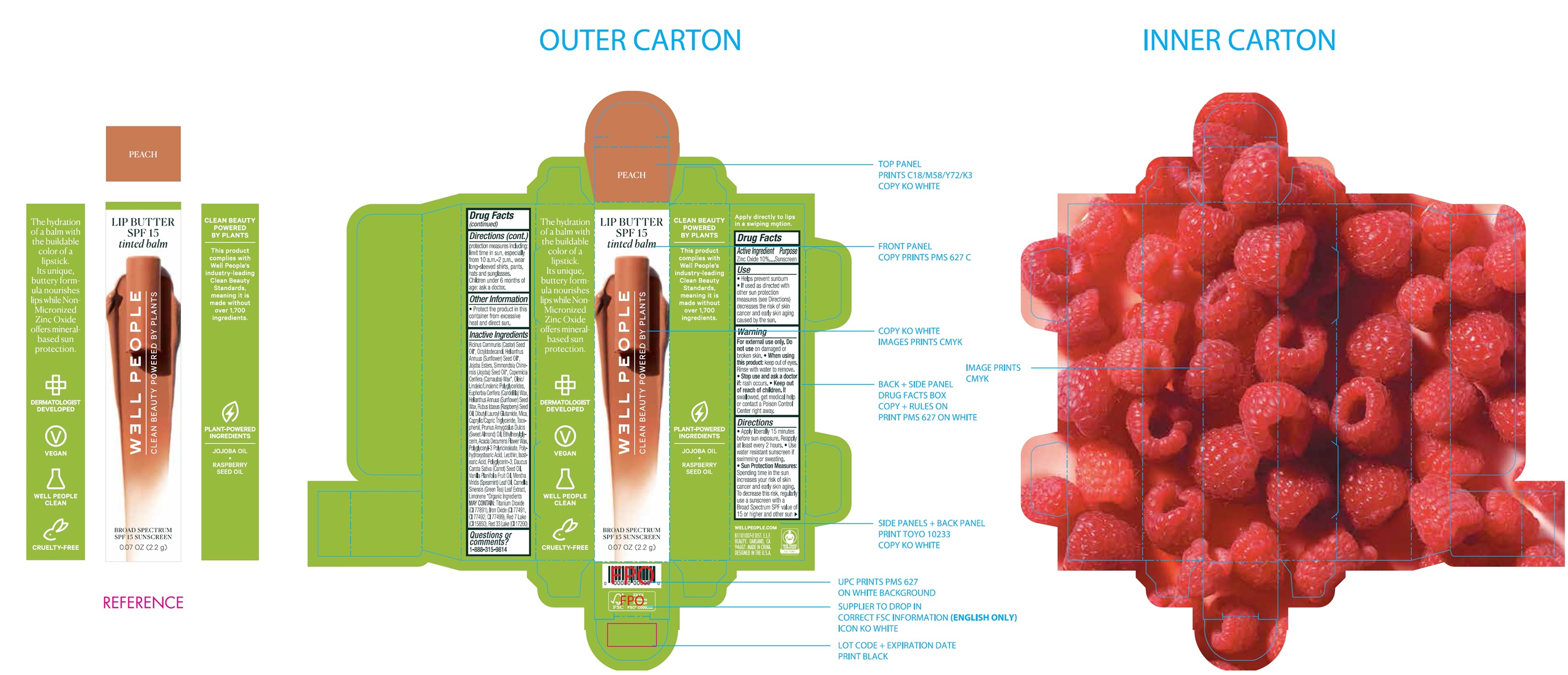
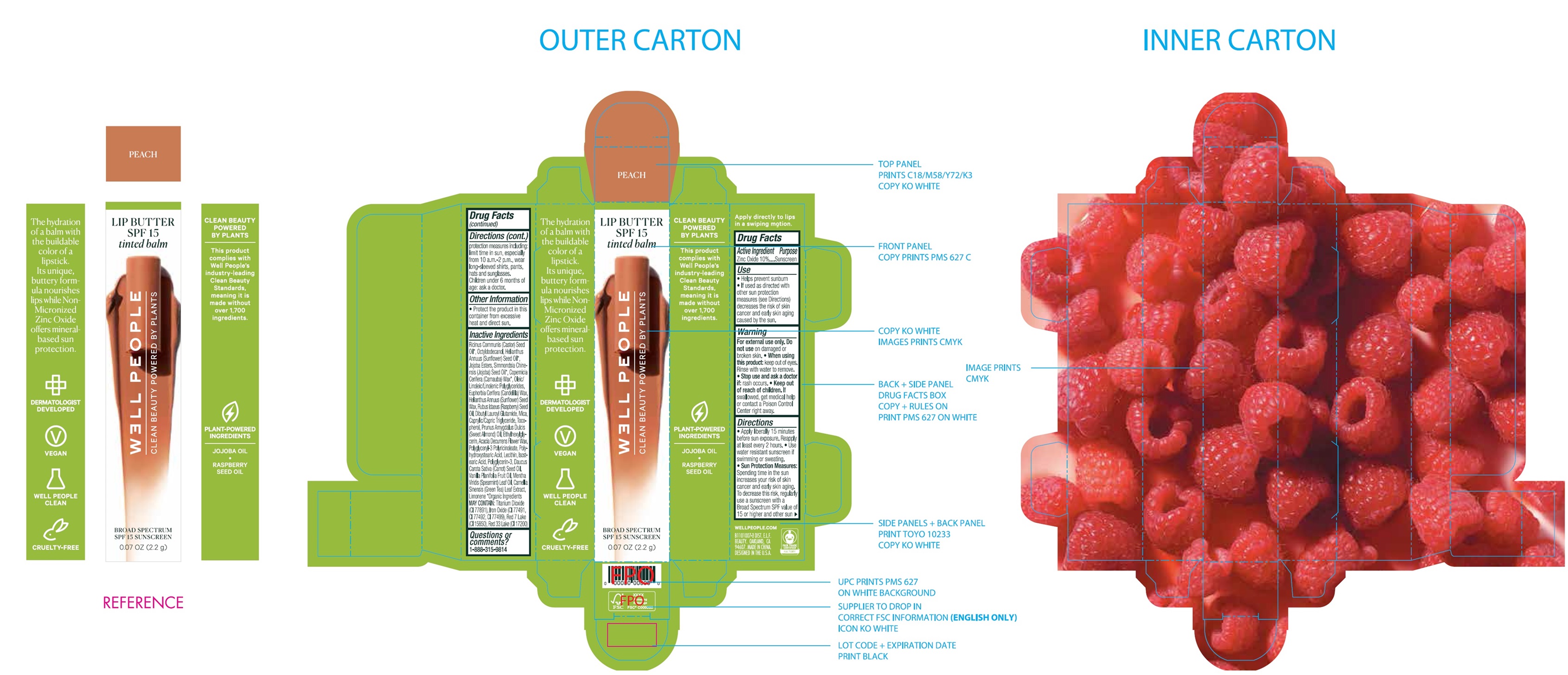
Label: WELL PEOPLE LIP BUTTER SPF 15 SUNSCREEN PEACH- zinc oxide cream
- NDC Code(s): 76354-492-01
- Packager: e.l.f. Cosmetics, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Use
- Warning
-
Directions
- Apply liberally 15 minutes before sun exposure. Reapply at least every 2 hours.
- Use water resistant sunscreen if swimming or sweating.
- Sun Protection Measures:Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:limit time in sun, especially from 10 a.m.-2 p.m., wear long-sleeved shirts, pants, hats and sunglasses. Children under 6 months of age: ask a doctor.
- Other Information
-
Inactive Ingredients
Ricinus Communis (Castor) Seed Oil*, Octyldodecanol, Heliathus Annuus (Sunflower) Seed Oil*, Jojoba Esters, Simmondsia Chinensis (Jojoba) Seed Oil*, Copernicia Cerifera (Carnauba) Wax*, Oleic/Linoleic/Linolenic Polyglycerides, Euphorbia Cerifera (Candelilla) Wax, Helianthus Annuus (Sunflower) Seed Wax, Rubus Idaeus (Raspberry) Seed Oil, Dibutyl Lauroyl Glutamide, Mica, Caprylic/Capric Triglyceride, Tocopherol, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Ethylhexylglycerin, Acacia Decurrens Flower Wax, Polyglyceryl-3 Polyricinoleate, Polyhydroxystearic Acid, Lecithin, Isostearic Acid, Polyglycerin-3, Daucus Carota Sativa (Carrot) Seed Oil, Vanilla Planifolia Fruit Oil, Mentha Viridis (Spearmint) Leaf Oil, Camellia Sinensis (Green Tea) Leaf Extract, Limonene *Orgainc Ingredients MAY CONTAIN:Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, CI 77492, CI 77499), Red 7 Lake (CI 15850), Red 33 Lake (CI 17200)
- Questions or comments?
- package Labeling:
-
INGREDIENTS AND APPEARANCE
WELL PEOPLE LIP BUTTER SPF 15 SUNSCREEN PEACH
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76354-492 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 100 mg in 1 g Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) OCTYLDODECANOL (UNII: 461N1O614Y) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) SUNFLOWER OIL (UNII: 3W1JG795YI) JOJOBA OIL (UNII: 724GKU717M) CARNAUBA WAX (UNII: R12CBM0EIZ) CANDELILLA WAX (UNII: WL0328HX19) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) RASPBERRY SEED OIL (UNII: 9S8867952A) DIBUTYL LAUROYL GLUTAMIDE (UNII: 3V7K3IA58X) MICA (UNII: V8A1AW0880) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) TOCOPHEROL (UNII: R0ZB2556P8) ALMOND OIL (UNII: 66YXD4DKO9) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ACACIA DECURRENS FLOWER WAX (UNII: AU6XZE9IY9) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) CARROT SEED OIL (UNII: 595AO13F11) VANILLA PLANIFOLIA OIL (UNII: 0A3F415158) SPEARMINT OIL (UNII: C3M81465G5) GREEN TEA LEAF (UNII: W2ZU1RY8B0) LIMONENE, (+)- (UNII: GFD7C86Q1W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76354-492-01 1 in 1 BOX 09/01/2021 1 2.2 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/01/2021 Labeler - e.l.f. Cosmetics, Inc (093902816)