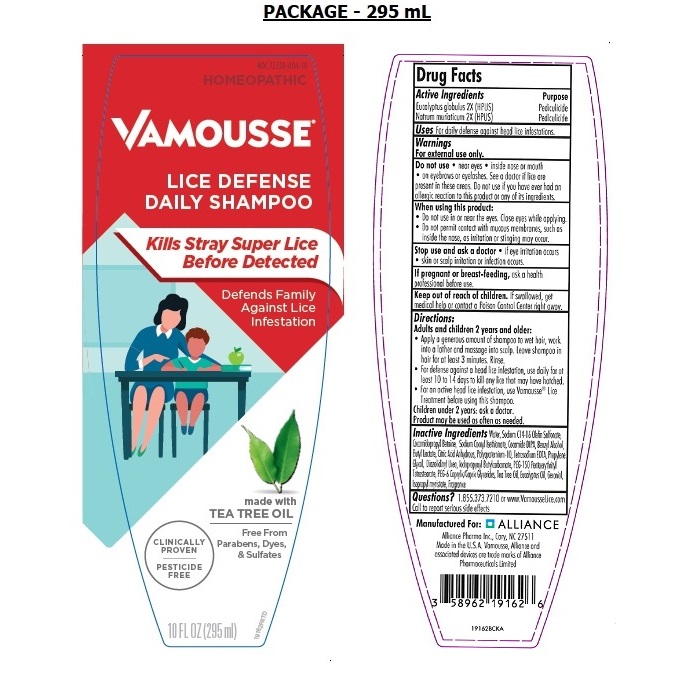
Label: VAMOUSSE LICE DEFENSE- eucalyptus globulus, natrum muriaticum shampoo
- NDC Code(s): 72238-004-01, 72238-004-02, 72238-004-03, 72238-004-05
- Packager: Alliance Pharma Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only.
Do not use• near eyes • inside nose or mouth • on eyebrows or eyelashes. See a doctor if lice are present in these areas. Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
When using this product:
• Do not use in or near the eyes. Close eyes while applying.
• Do not permit contact with mucous membranes, such as inside the nose, as irritation or stinging may occur.
Stop use and ask a doctor• if eye irritation occurs • skin or scalp irritation or infection occurs.
If pregnant or breast-feeding,ask a health professional before use.
-
Directions:
Adults and children 2 years and older:
• Apply a generous amount of shampoo to wet hair, work into a lather and massage into scalp. Leave shampoo in hair for at least 3 minutes. Rinse.
• For defense against a head lice infestation, use daily for at least 10 to 14 days to kill any lice that may have hatched.
• For an active head lice infestation, use Vamousse ®Lice Treatment before using this shampoo.
Children under 2 years: ask a doctor.
Product may be used as often as needed.
-
Inactive Ingredients
Water, Sodium C14-16 Olefin Sulfonate, Cocamidopropyl Betaine, Sodium Cocoyl Isethionate, Cocamide DIPA, Benzyl Alcohol, Butyl Lactate, Citric Acid Anhydrous, Polyquaternium-10, Tetrasodium EDTA, Propylene Glycol, Diazolidinyl Urea, Iodopropynyl Butylcarbamate, PEG-150 Pentaerythrityl Tetrastearate, PEG-6 Caprylic/Capric Glycerides, Tea Tree Oil, Eucalyptus Oil, Geraniol, Isopropyl myristate, Fragrance
- Questions?
-
SPL UNCLASSIFIED SECTION
 NEW
NEW
PESTICIDE - FREE
Kill Stray Super Lice*
Before Detected
with Tea Tree Oil
Defends Family Against Lice Infestation
CLINICALLY PROVEN
Use After Exposure or Treatment
Follow-up with Favorite Conditioner
No Parabens, Sulfates or Dyes
HOMEOPATHIC
*As shown in lab studies
Manufactured for: ALLIANCE
Alliance Pharma Inc., Cary, NC 27518
Made in the U.S.A Vamousse, Alliance and associated devices are trade marks of Alliance Pharmaceuticals Limited
- Packaging
-
INGREDIENTS AND APPEARANCE
VAMOUSSE LICE DEFENSE
eucalyptus globulus, natrum muriaticum shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72238-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) (EUCALYPTUS GLOBULUS LEAF - UNII:S546YLW6E6) EUCALYPTUS GLOBULUS LEAF 2 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 2 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SODIUM COCOYL ISETHIONATE (UNII: 518XTE8493) PEG-150 PENTAERYTHRITYL TETRASTEARATE (UNII: 8L4OOQ76AM) CAPRYLOCAPROYL POLYOXYLGLYCERIDES 6 (UNII: GO50W2HWO8) COCO DIISOPROPANOLAMIDE (UNII: S485AM948Q) TEA TREE OIL (UNII: VIF565UC2G) BENZYL ALCOHOL (UNII: LKG8494WBH) EUCALYPTUS OIL (UNII: 2R04ONI662) BUTYL LACTATE (UNII: 0UI63W814U) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) POLYQUATERNIUM-10 (125 MPA.S AT 2%) (UNII: L45WU8S981) GERANIOL (UNII: L837108USY) EDETATE SODIUM (UNII: MP1J8420LU) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72238-004-01 295 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2021 2 NDC:72238-004-03 400 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2021 3 NDC:72238-004-05 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2021 4 NDC:72238-004-02 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/01/2021 Labeler - Alliance Pharma Inc. (081138954)