Label: EPSOM SALT granule
- NDC Code(s): 50804-395-01, 50804-395-04
- Packager: Good Sense (Geiss, Destin & Dunn, Inc.)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
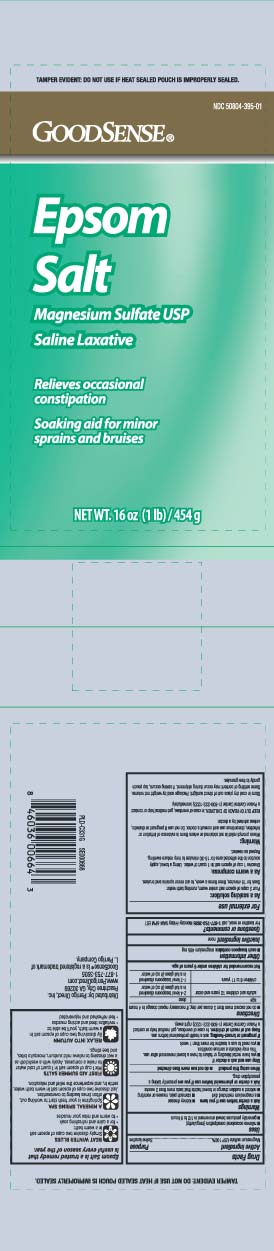
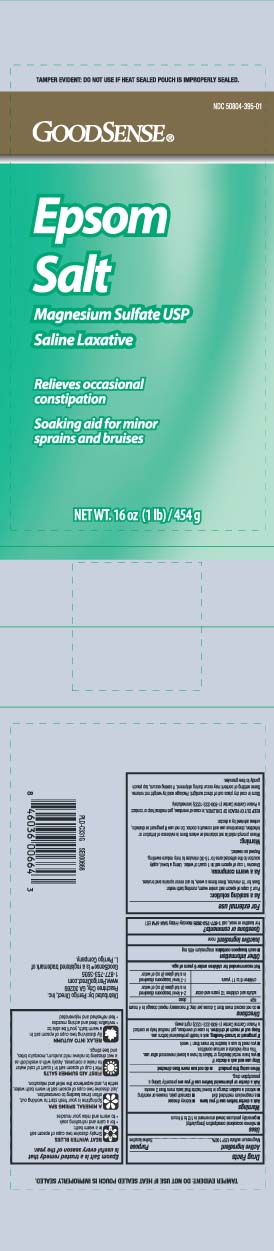
Active ingredientMagnesium sulfate USP 100%
-
PurposeSaline laxative
-
Usesrelieves occasional constipation (irregularity) generally produces bowel movement in 1/2 to 6 hours
-
WarningsAsk a doctor before use if you have - kidney disease - a magnesium restricted diet - stomach pain, nausea or vomiting - noticed a sudden change in bowel habits that lasts more than 2 weeks - Ask ...
-
Directionsdo not exceed more than 2 doses per day; if necessary repeat dosage in 4 hours - agedose - adults and children 12 years and older2-4 level teaspoons dissolved in a full glass (8oz) of ...
-
Other informationeach teaspoon contains: magnesium 495 mg
-
Inactive ingredientnone
-
Questions or comments?For laxative or soak, call 1-877-753-3935 Monday- Friday 9AM-5PM EST
-
Principal Display PanelEPSOM SALTS - Magnesium Sulfate USP - Saline Laxative - Relieves occasional constipation - Soaking aod for minor sprains and bruises - Net Wt LB (kg) TAMPER EVIDENT: DO NOT USE IF HEAT SEALED POUCH IS ...
-
Package LabelGOOD SENSE Epsom Salt
-
INGREDIENTS AND APPEARANCEProduct Information