Label: BIVALIRUDIN injection, solution
- NDC Code(s): 70511-142-50, 70511-142-84
- Packager: MAIA Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BIVALIRUDIN INJECTION safely and effectively. See full prescribing information for BIVALIRUDIN INJECTION. BIVALIRUDIN injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEBivalirudin Injection is indicated for use as an anticoagulant in patients undergoing percutaneous coronary intervention (PCI), including patients with heparin-induced thrombocytopenia and ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dose of Bivalirudin Injection is an intravenous bolus dose of 0.75 mg/kg, followed immediately by a maintenance infusion of 1.75 mg/kg/h for the duration ...
-
3 DOSAGE FORMS AND STRENGTHSInjection, clear to slightly opalescent, colorless to yellow sterile solution: 250 mg of bivalirudin per 50 mL (5 mg/mL) in a single-dose vial. Ready-to-use. Each vial contains 250 mg of ...
-
4 CONTRAINDICATIONSBivalirudin Injection is contraindicated in patients with: Significant active bleeding; Hypersensitivity to Bivalirudin Injection or its components [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS5.1 Bleeding Events - Bivalirudin increases the risk of bleeding [see Adverse Reactions (6.1)]. Bivalirudin’s anticoagulant effect subsides approximately one hour after discontinuation [see ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSIn clinical trials in patients undergoing PCI, co-administration of bivalirudin with heparin, warfarin, thrombolytics, or GPIs was associated with increased risks of major bleeding events compared ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on use of bivalirudin in pregnant women to inform a drug-associated risk of adverse developmental outcomes. Reproduction studies in rats ...
-
10 OVERDOSAGECases of overdose of up to 10 times the recommended bolus or continuous infusion dose of bivalirudin have been reported in clinical trials and in postmarketing reports. A number of the reported ...
-
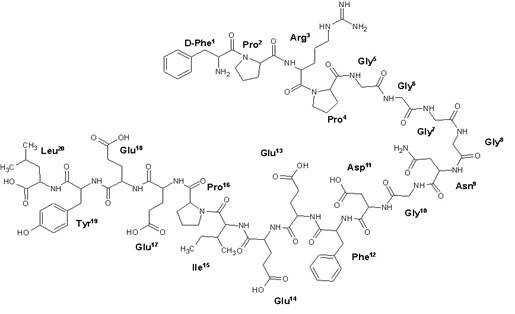
11 DESCRIPTIONBivalirudin Injection contains bivalirudin trifluoroacetate, which is a specific and reversible direct thrombin inhibitor. Bivalirudin trifluoroacetate is a synthetic, 20 amino acid peptide salt ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Bivalirudin directly inhibits thrombin by specifically binding both to the catalytic site and to the anion-binding exosite of circulating and clot-bound thrombin ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No long-term studies in animals have been performed to evaluate the carcinogenic potential of bivalirudin. Bivalirudin displayed no ...
-
14 CLINICAL STUDIESBivalirudin Angioplasty Trial (BAT) In the BAT studies, patients with unstable angina undergoing PCI were randomized 1:1 to a 1 mg/kg bolus of bivalirudin and then 2.5 mg/kg/h for four hours ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Bivalirudin Injection is supplied as a refrigerated, ready-to-use, clear to slightly opalescent, colorless to yellow, sterile solution in 250 mg/50 mL (5 mg/mL) single-dose ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to watch carefully for any signs of bleeding or bruising and to report these to their healthcare provider when they occur. Manufactured for - MAIA Pharmaceuticals, Inc. Princeton, NJ ...
-
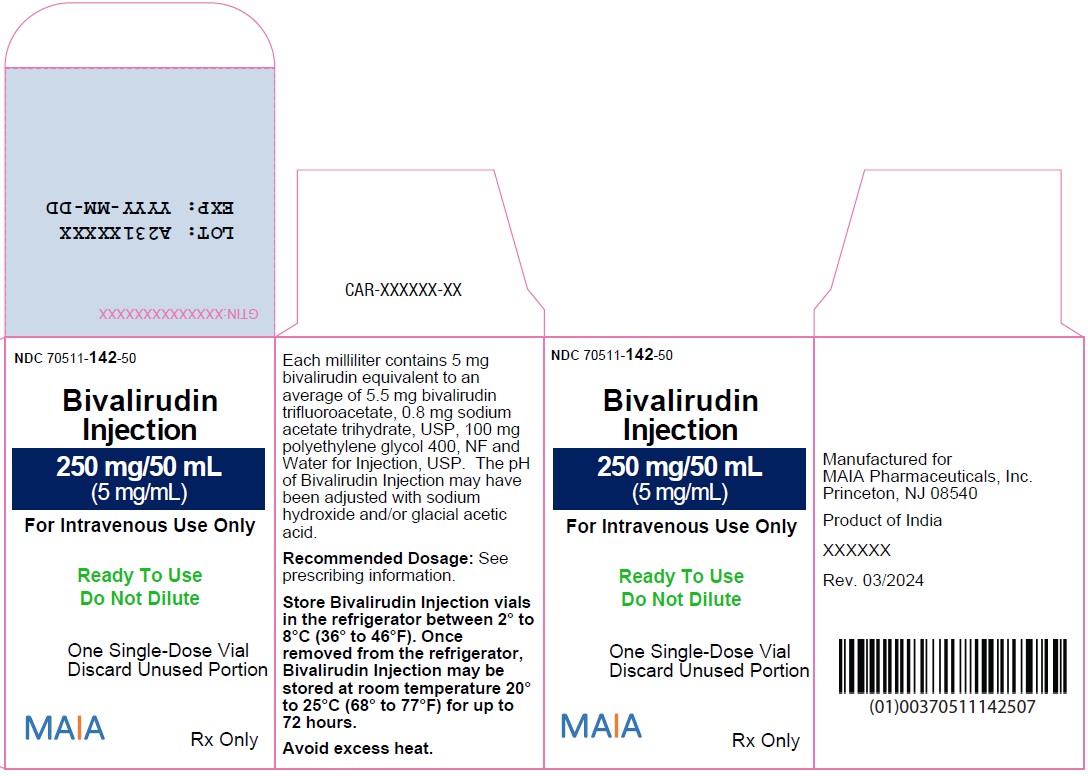
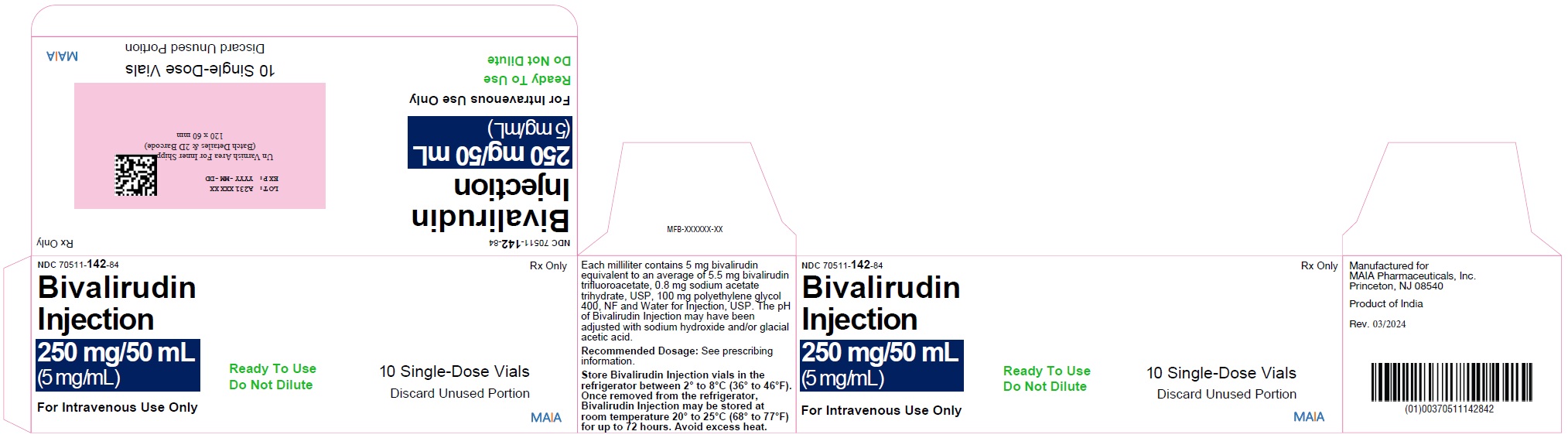
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70511-142-50 - Bivalirudin - Injection - 250 mg/50 mL - (5 mg/mL) For Intravenous Use Only - Ready to Use - Do Not Dilute - One Single-Dose Vial - Discard Unused Portion - MAIA Rx Only - NDC 70511-142-84 ...
-
INGREDIENTS AND APPEARANCEProduct Information