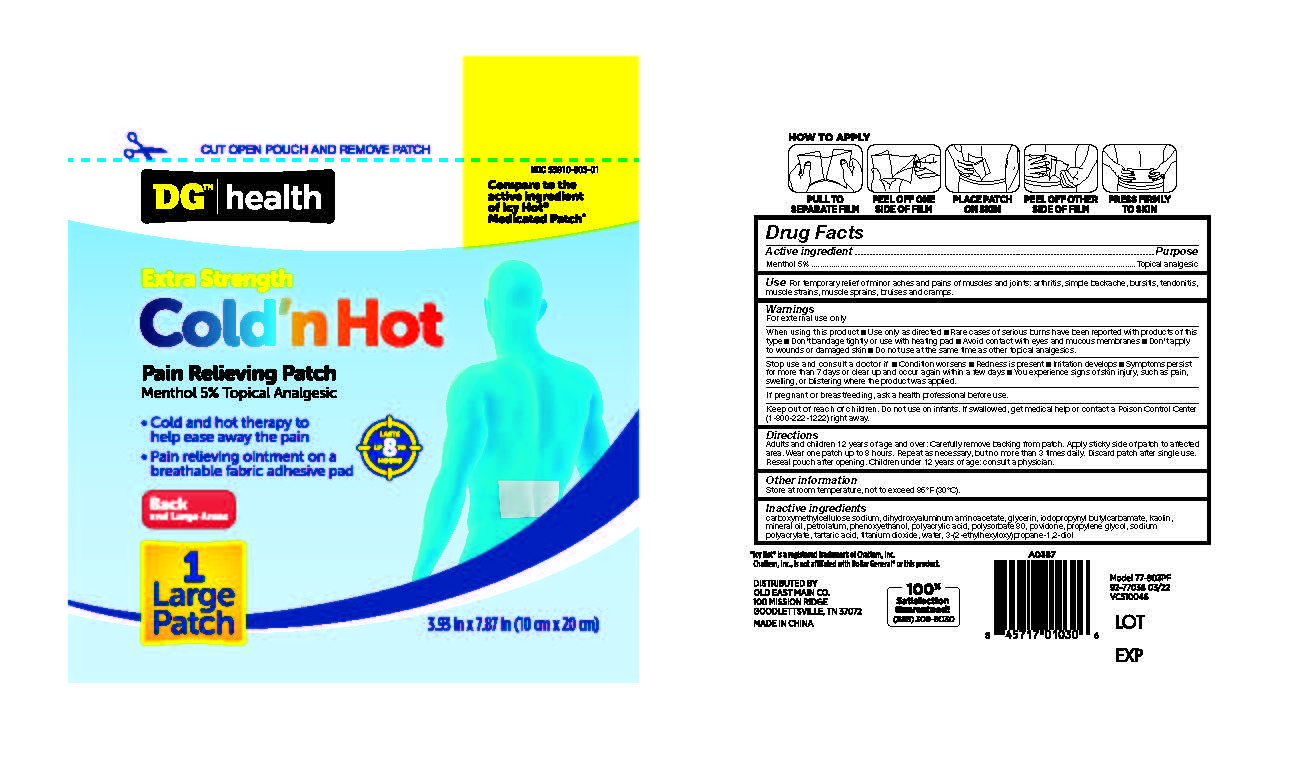
Label: DOLLAR GENERAL COLD-HOT MEDICATED PATCHES- menthol patch
- NDC Code(s): 55910-903-01
- Packager: DOLGENCORP, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
carboxymethylcellulose sodium, Dihydroxyaluminum Aminoacetate, Glycerin, iodopropynyl butylcarbamate, Kaolin, Mineral Oil, Petrolatum, Phenoxyethanol, Polyacrylic Acid, Polysorbate 80, povidone, Propylene Glycol, Sodium Polyacrylate, Tartaric Acid, Titanium Dioxide, Water, 3-(2-ethylhexyloxy)propane-1,2-diol
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- PURPOSE
- When using this product
- Stop use and ask a doctor
- If pregnant or breastfeeding
- Dollar General Cold-Hot Medicated Patches
-
INGREDIENTS AND APPEARANCE
DOLLAR GENERAL COLD-HOT MEDICATED PATCHES
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55910-903 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) WATER (UNII: 059QF0KO0R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PHENOXYETHANOL (UNII: HIE492ZZ3T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) KAOLIN (UNII: 24H4NWX5CO) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TARTARIC ACID (UNII: W4888I119H) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) POLYACRYLIC ACID (8000 MW) (UNII: 73861X4K5F) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55910-903-01 1 in 1 BOX 10/01/2017 1 1 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/01/2017 Labeler - DOLGENCORP, LLC (068331990)