EPINEPHRINE injection (ep-in-eph-rine),
for intramuscular or subcutaneous use
- For allergic emergencies (anaphylaxis)
Read this Patient Information leaflet carefully before you use epinephrine ...
EPINEPHRINE injection (ep-in-eph-rine),
for intramuscular or subcutaneous use
For allergic emergencies (anaphylaxis)
Read this Patient Information leaflet carefully before you use epinephrine injection, and each time you get a refill. There may be new information. You, your parent, caregiver, or others who may be in a position to administer epinephrine injection should know how to use it before you have an allergic emergency.
This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about epinephrine injection?
1. Epinephrine injection contains epinephrine, a medicine used to treat allergic emergencies (anaphylaxis). Anaphylaxis can be life-threatening, can happen within minutes, and can be caused by stinging and biting insects, allergy injections, foods, medicines, exercise, or other unknown causes. Symptoms of an anaphylaxis may include:
- trouble breathing
- wheezing
- hoarseness (changes in the way your voice sounds)
- hives (raised reddened rash that may itch)
- severe itching
- swelling of your face, lips, mouth, or tongue
- skin rash, redness, or swelling
- fast heartbeat
- weak pulse
- feeling very anxious
- confusion
- stomach pain
- losing control of urine or bowel movements (incontinence)
- diarrhea or stomach cramps
- dizziness, fainting, or “passing out” (unconsciousness)
2. Always carry your epinephrine injection with you because you may not know when anaphylaxis may happen. Talk to your healthcare provider if you need additional units to keep at work, school, or other locations. Tell your family members, caregivers, and others where you keep your epinephrine injection and how to use it before you need it. You may be unable to speak in an allergic emergency.
3. When you have an allergic emergency (anaphylaxis)
-
Use epinephrine injection right away.
-
Get emergency medical help right away. You may need further medical attention. You may need to use a second epinephrine injection if symptoms continue or recur. Only a healthcare provider should give additional doses of epinephrine if you need more than 2 injections for a single anaphylaxis episode.
What is epinephrine injection?
- Epinephrine injection is a disposable, prefilled automatic injection device (auto-injector) used to treat life-threatening, allergic emergencies including anaphylaxis in people who are at risk for or have a history of serious allergic emergencies. Each device contains a single dose of epinephrine.
- Epinephrine injection is for immediate self (or caregiver) administration and does not take the place of emergency medical care. You should get emergency medical help right away after using epinephrine injection.
- Epinephrine injection is for people who have been prescribed this medicine by their healthcare provider.
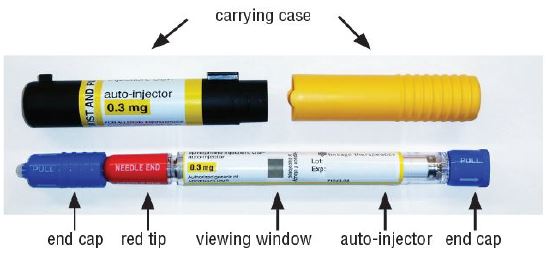
- The epinephrine injection 0.3 mg auto-injector is for patients who weigh 66 pounds or more (30 kilograms or more).
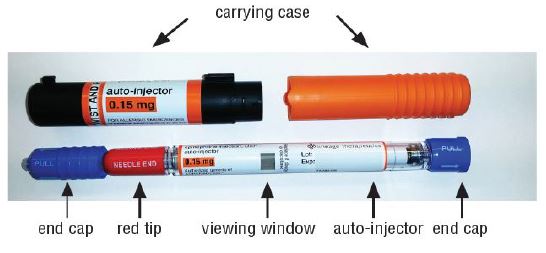
- The epinephrine injection 0.15 mg auto-injector is for patients who weigh about 33 to 66 pounds (15 to 30 kilograms).
- It is not known if epinephrine injection is safe and effective in children who weigh less than 33 pounds (15 kilograms).
Before using epinephrine injection, tell your healthcare provider about all your medical conditions, especially if you:
- have heart problems or high blood pressure
- have diabetes
- have thyroid problems
- have asthma
- have a history of depression
- have Parkinson's disease
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if epinephrine will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if epinephrine passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Tell your healthcare provider of all known allergies.
Especially tell your healthcare provider if you take certain asthma medicines.
Epinephrine injection and other medicines may affect each other, causing side effects. Epinephrine injection may affect the way other medicines work, and other medicines may affect how epinephrine injection works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
Use your epinephrine injection for treatment of anaphylaxis as prescribed by your healthcare provider, regardless of your medical conditions or the medicine you take.
How should I use epinephrine injection?
- Each epinephrine injection contains only 1 dose of medicine.
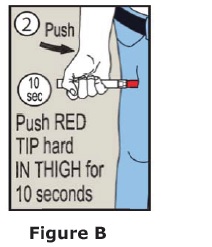
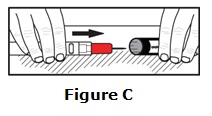
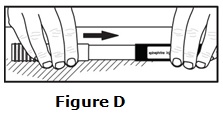
- Epinephrine injection should only be injected into the middle of the outer thigh (upper leg). It can be injected through clothing, if needed.
- Read the Instructions for Use at the end of this Patient Information Leaflet for information about the right way to use epinephrine injection.
- Your healthcare provider will show you how to safely use epinephrine injection.
- Use epinephrine injection exactly as your healthcare provider tells you to use it. You may need to use a second epinephrine injection if symptoms continue or recur. Only a healthcare provider should give additional doses of epinephrine if you need more than 2 injections for a single anaphylaxis episode.
-
Caution: Never put your thumb, fingers, or hand over the red tip. Never press or push the red tip with your thumb, fingers, or hand. The needle comes out of the red tip. Accidental injection into finger, hands, or feet may cause a loss of blood flow to those areas.
If this happens, go immediately to the nearest emergency room. Tell the healthcare provider where on your body you received the accidental injection.
- Your epinephrine injection comes packaged in a carton containing 1 or 2 epinephrine injection.
- You may request a separate Trainer, that comes packaged with instructions. Additional video instructions on the use of epinephrine injection are available from
www.epinephrineautoinject.com.
The epinephrine injection, USP auto-injector Trainer has a beige color. The beige epinephrine injection Trainer contains no medicine and no needle. Practice with your epinephrine injection Trainer before an allergic emergency happens to make sure you are able to safely use the real epinephrine injection in an emergency. Always carry your real epinephrine injection with you in case of an allergic emergency.
- Do not drop the carrying case or epinephrine injection. If the carrying case or epinephrine injection is dropped, check for damage and leakage. Throw away (dispose of) epinephrine injection and the carrying case, and replace if damage or leakage is noticed or suspected.
What are the possible side effects of epinephrine injection?
Epinephrine injection may cause serious side effects.
-
Epinephrine injection should only be injected into the middle of your outer thigh (upper leg). Do not inject epinephrine injection into your:
- veins
- buttocks
- fingers, toes, hands or feet.
If you accidentally inject epinephrine injection into any other part of your body, go to the nearest emergency room right away. Tell the healthcare provider where on your body you received the accidental injection.
Common side effects of epinephrine injection include:
- faster, irregular or “pounding” heartbeat
- sweating
- headache
- weakness
- shakiness
- paleness
- feelings of over excitement, nervousness, or anxiety
- dizziness
- nausea or vomiting
- breathing problems
These side effects may go away with rest.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of epinephrine injection. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store epinephrine injection?
- Store epinephrine injection at room temperature between 68°F to 77° F (20°C to 25° C).
- Protect from light.
-
Do not expose to extreme heat or cold. For example,
do not store in your vehicle’s glove box and
do not store in the refrigerator or freezer.
- Examine the contents in the clear viewing window of your epinephrine injection periodically. The solution should be clear. If the solution is discolored (pinkish or brown), cloudy or contains solid particles, replace the unit.
- Always keep your epinephrine injection in the carrying case to protect it from damage. The carrying case is not waterproof.
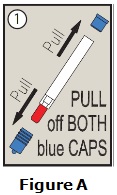
- The two blue end caps help to prevent accidental injection. Do not remove the blue end caps until you are ready to use epinephrine injection.
- Your epinephrine injection has an expiration date. Replace it before the expiration date.
- Throw away (dispose of) expired, unwanted, or unused epinephrine injections in an FDA-cleared sharps disposal container. Do not throw away epinephrine injection in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- Made of heavy-duty plastic,
- Can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- Upright and stable during use,
- Leak-resistant, and
- Properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposalVisit the FDA’s website (https://www.fda.gov/drugs/safe-disposal-medicines/disposal- unused-medicines-what-you-should-know) for more information about how to throw away (dispose of) unused, unwanted or expired medicines.
Keep epinephrine injection and all medicines out of the reach of children.
General information about the safe and effective use of epinephrine injection:
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use epinephrine injection for a condition for which it was not prescribed. Do not give epinephrine injection to other people.
This Patient Information Leaflet summarizes the most important information about epinephrine injection. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about epinephrine injection that is written for health professionals.
What are the ingredients in epinephrine injection?
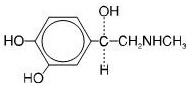
Active Ingredient: epinephrine
Inactive Ingredients: sodium chloride, chlorobutanol, sodium bisulfite, hydrochloric acid and sodium hydroxide, and water.
For more information and video instructions on the use of epinephrine injection go to www.epinephrineautoinject.com or call 1-877-835-5472.
Important Information
-
The epinephrine injection 0.3 mg has a yellow colored label.
-
The epinephrine injection 0.15 mg has an orange colored label.
-
The epinephrine injection Trainer has a beige color, and contains no medicine and no needle.
-
Your epinephrine injection is designed to work through clothing.
-
The two blue end caps on epinephrine injection help to prevent accidental injection of the device. Do not remove the blue end caps until you are ready to use it.
-
Only inject into the middle of the outer thigh (upper leg). Never inject into any other part of the body.
-
Never put your thumb, fingers, or your hand over the red tip. The needle comes out of the red tip.
-
If an accidental injection happens, get medical help right away.
-
Do not place patient information or any other foreign objects in carrier with the epinephrine injection, as this may prevent you from removing the auto-injector for use.
This Patient Information has been approved by the U.S. Food and Drug Administration Rev. 02-2021-03
Close