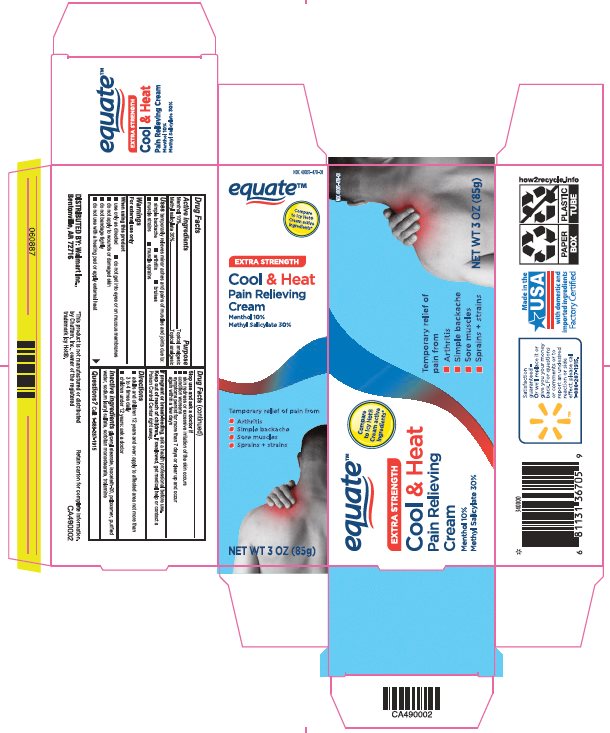
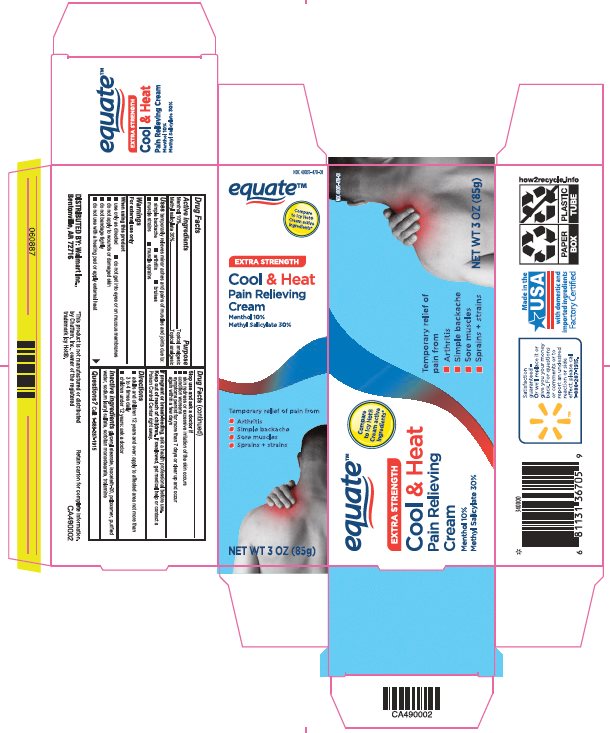
Label: EQUATE COOL AND HEAT- menthol, methyl salicylate cream
- NDC Code(s): 49035-470-01
- Packager: Wal-Mart Stores Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- If pregnant or breast-feeding,
- Keep Out of Reach of Children
- Directions
- Inactive Ingredients
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
EQUATE COOL AND HEAT
menthol, methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-470 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 100 mg in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 300 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ISOCETETH-20 (UNII: O020065R7Z) POLOXAMER 407 (UNII: TUF2IVW3M2) WATER (UNII: 059QF0KO0R) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-470-01 1 in 1 CARTON 09/17/2018 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/17/2018 Labeler - Wal-Mart Stores Inc (051957769)