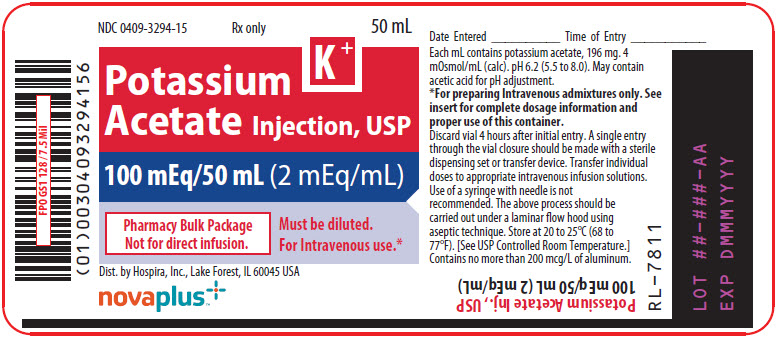
Label: POTASSIUM ACETATE injection, solution, concentrate
- NDC Code(s): 0409-3294-15, 0409-3294-25
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Potassium Acetate Injection, USP (2 mEq/mL) is a sterile, nonpyrogenic, concentrated solution of potassium acetate in water for injection. The solution is administered after dilution by the intravenous route as an electrolyte replenisher. It must not be administered undiluted.
Each mL contains 196 mg of potassium acetate which provides 2 mEq each of potassium (K+) and acetate (CH3COO¯). The solution may contain acetic acid for pH adjustment. The pH is 6.2 (range: 5.5 to 8.0). The osmolar concentration is 4 mOsmol/mL (calc.); specific gravity is 1.089.
The Pharmacy Bulk Package is a sterile dosage which contains multiple single doses for use only in a pharmacy bulk admixture program.
The solution is intended as an alternative to potassium chloride to provide potassium ion (K+) for addition to large volume infusion fluids for intravenous use.
Potassium Acetate, USP is chemically designated CH3COOK, and is comprised of colorless crystals or a white crystalline powder that is very soluble in water.
The Pharmacy Bulk Package is designed for use with manual, gravity flow operations and automated compounding devices for preparing sterile parenteral nutrient admixtures. The Pharmacy Bulk Package contains no bacteriostat, antimicrobial agent, or added buffer. Multiple single doses may be dispensed during continual aliquoting operations.
-
CLINICAL PHARMACOLOGY
As the principal cation of the intracellular fluid, potassium plays an important role in fluid and electrolyte balance. The normal potassium concentration in the intracellular fluid compartment is about 160 mEq/liter. The normal serum potassium range is 3.5 to 5.0 mEq/liter. The kidney normally regulates potassium balance but does not conserve potassium as well or as promptly as it conserves sodium. The daily turnover of potassium in the normal adult averages 50 to 150 mEq (milliequivalents) and represents 1.5 to 5% of the total potassium content of the body.
Acetate (CH3COO¯), a source of hydrogen ion acceptors, is an alternate source of bicarbonate (HCO3¯) by metabolic conversion in the liver. This has been shown to proceed readily, even in the presence of severe liver disease.
-
INDICATIONS AND USAGE
Potassium Acetate Injection, USP (2 mEq/mL) is indicated as a source of potassium, for the addition to large volume intravenous fluids, to prevent or correct hypokalemia in patients with restricted or no oral intake. It is also useful as an additive for preparing specific intravenous fluid formulas when the needs of the patient cannot be met by standard electrolyte or nutrient solutions.
- CONTRAINDICATIONS
-
WARNINGS
Potassium Acetate Injection, USP (2 mEq/mL) must be diluted before use.
To avoid potassium intoxication, infuse potassium-containing solutions slowly. Potassium replacement therapy should be monitored whenever possible by continuous or serial electrocardiography (ECG). Serum potassium levels are not necessarily dependable indicators of tissue potassium levels.
Solutions which contain potassium ions should be used with great care, if at all, in patients with hyperkalemia, severe renal failure and in conditions in which potassium retention is present.
In patients with diminished renal function, administration of solutions containing potassium ions may result in potassium retention.
Solutions containing acetate ions should be used with great care in patients with metabolic or respiratory alkalosis. Acetate should be administered with great care in those conditions in which there is an increased level or an impaired utilization of this ion, such as severe hepatic insufficiency.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
PRECAUTIONS
Do not administer unless solution is clear and seal is intact. Discard unused portion.
Potassium replacement therapy should be guided primarily by ECG monitoring and secondarily by the serum potassium level.
High plasma concentrations of potassium may cause death by cardiac depression, arrhythmias or arrest.
Use with caution in the presence of cardiac disease, particularly in digitalized patients or in the presence of renal disease.
Solutions containing acetate ion should be used with caution as excess administration may result in metabolic alkalosis.
Pregnancy
Animal reproduction studies have not been conducted with potassium acetate. It is also not known whether potassium acetate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Potassium acetate should be given to a pregnant woman only if clearly needed.
Pediatric Use: The safety and effectiveness of potassium acetate have been established in pediatric patients.
Geriatric Use: An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Potassium ions are known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Adverse reactions involve the possibility of potassium intoxication. The signs and symptoms of potassium intoxication include paresthesias of the extremities, flaccid paralysis, listlessness, mental confusion, weakness and heaviness of the legs, hypotension, cardiac arrhythmias, heart block, electrocardiographic abnormalities such as disappearance of P waves, spreading and slurring of the QRS complex with development of a biphasic curve and cardiac arrest. (See WARNINGS and PRECAUTIONS.)
-
OVERDOSAGE
In the event of overdosage, discontinue infusion containing potassium acetate immediately and institute corrective therapy as indicated to reduce elevated serum potassium levels and restore acid-base balance if necessary. (See WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS.)
-
DOSAGE AND ADMINISTRATION
Potassium Acetate Injection, USP (2 mEq/mL) in the Pharmacy Bulk Package is designed for use with manual, gravity flow operations and automated compounding devices for preparing sterile nutrient admixtures.
Potassium Acetate Injection, USP (2 mEq/mL) is administered intravenously only after dilution in a larger volume of fluid. The dose and rate of administration are dependent upon the individual needs of the patient. ECG and serum potassium should be monitored as a guide to dosage. Using aseptic technique, all or part of the contents of one or more vials may be added to other intravenous fluids to provide any desired number of milliequivalents (mEq) of potassium (K+) with an equal number of milliequivalents of acetate (CH3COO¯).
Maximum infusion rate: The infusion rate should not exceed 1 mEq/kg/hr.
Normal daily requirements:
Newborn: 2–6 mEq/kg/24 hr.
Children: 2–3 mEq/kg/24 hr.
Adult: 40 – 80 mEq/24 hr.Intraosseous infusion can be an alternate route for drug administration when intravenous access is not readily available.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. (See PRECAUTIONS.)
Directions for Dispensing From Pharmacy Bulk Package
The Pharmacy Bulk Package is for use in the Pharmacy Admixtures Service only. For hanger application, peel off the paper liner from both ends of the tape hanger to expose 3/4 inch long adhesive portions. Adhere each end to the label on the bottle. The vials should be suspended as a unit in the laminar flow hood.
A single entry through the vial closure should be made with a sterile dispensing set or transfer device. Transfer individual doses to appropriate intravenous infusion solutions. Use of a syringe with needle is not recommended as it may cause leakage and multiple entries will increase the potential of microbial and particulate contamination.
The above process should be carried out under a laminar flow hood using aseptic technique. Discard any unused portion within 4 hours after initial closure entry.
-
HOW SUPPLIED
Potassium Acetate Injection, USP is a Pharmacy Bulk Package which provides multiple single doses for continuous admixture compounding procedures is supplied as follows:
Unit of Sale Concentration Each NDC 0409-3294-25
100 mEq/50 mL
NDC 0409-3294-15
Tray of 25
(2 mEq/mL)
Glass Fliptop Vial
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
Novaplus is a registered trademark of Vizient, Inc.
LAB-0930-4.0
Revised: 11/2020
- PRINCIPAL DISPLAY PANEL - 50 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 50 mL Vial Tray
-
INGREDIENTS AND APPEARANCE
POTASSIUM ACETATE
potassium acetate injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0409-3294 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM ACETATE (UNII: M911911U02) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM ACETATE 196.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0409-3294-25 25 in 1 TRAY 12/23/2013 1 NDC:0409-3294-15 50 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018896 12/23/2013 Labeler - Hospira, Inc. (141588017) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 093132819 ANALYSIS(0409-3294) , MANUFACTURE(0409-3294) , PACK(0409-3294) , LABEL(0409-3294) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 827731089 ANALYSIS(0409-3294) Establishment Name Address ID/FEI Business Operations Pfizer Healthcare India Private Limited 860037912 ANALYSIS(0409-3294) , MANUFACTURE(0409-3294) , PACK(0409-3294) , LABEL(0409-3294)