Label: TICAGRELOR tablet, film coated
- NDC Code(s): 42658-115-03, 42658-115-07, 42658-115-10
- Packager: Hisun Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TICAGRELOR TABLETS safely and effectively. See full prescribing information for TICAGRELOR TABLETS. TICAGRELOR tablets, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: BLEEDING RISK
- Ticagrelor, like other antiplatelet agents, can cause significant, sometimes fatal bleeding ( 5.1, 6.1>).
- Do not use ticagrelor in patients with active pathological bleeding or a history of intracranial hemorrhage ( 4.1, 4.2).
- Do not start ticagrelor in patients undergoing urgent coronary artery bypass graft surgery (CABG) ( 5.1, 6.1).
If possible, manage bleeding without discontinuing ticagrelor. Stopping ticagrelor increases the risk of subsequent cardiovascular events ( 5.2).
Close -
1 INDICATIONS AND USAGE1.2 Coronary Artery Disease but No Prior Stroke or Myocardial Infarction - Ticagrelor is indicated to reduce the risk of a first MI or stroke in patients with coronary artery disease (CAD) at ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Instructions - Advise patients who miss a dose of ticagrelor tablets to take their next dose at its scheduled time. For patients who are unable to swallow tablets whole, ticagrelor ...
-
3 DOSAGE FORMS AND STRENGTHSTicagrelor tablets 90 mg are supplied as a round, biconvex, yellow, film-coated tablet debossed with a “HU” on one side and “90” on the other side.
-
4 CONTRAINDICATIONS4.1 History of Intracranial Hemorrhage - Ticagrelor is contraindicated in patients with a history of intracranial hemorrhage (ICH) because of a high risk of recurrent ICH in this population ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Bleeding - Drugs that inhibit platelet function including ticagrelor increase the risk of bleeding - [see Warnings and Precautions ( 5.2) and Adverse Reactions ( 6.1)] ...
-
6 ADVERSE REACTIONSThe following adverse reactions are also discussed elsewhere in the labeling: Bleeding - [see Warnings and Precautions ( 5.1)] Dyspnea - [see Warnings and Precautions ( 5.3) ...
-
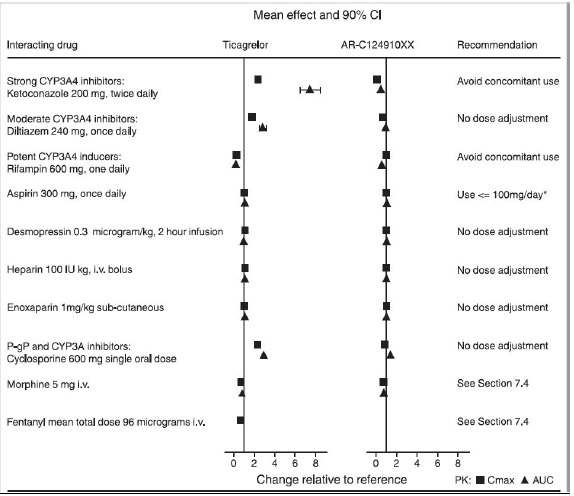
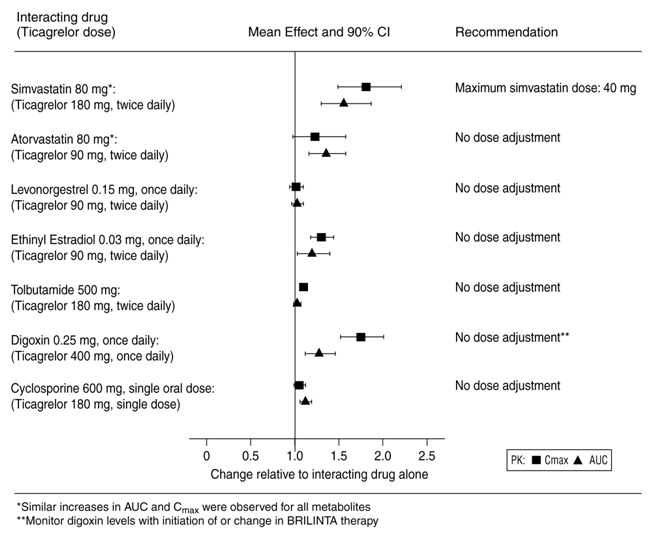
7 DRUG INTERACTIONS7.1 Strong CYP3A Inhibitors - Strong CYP3A inhibitors substantially increase ticagrelor exposure and so increase the risk of dyspnea, bleeding, and other adverse events. Avoid use of strong ...
-
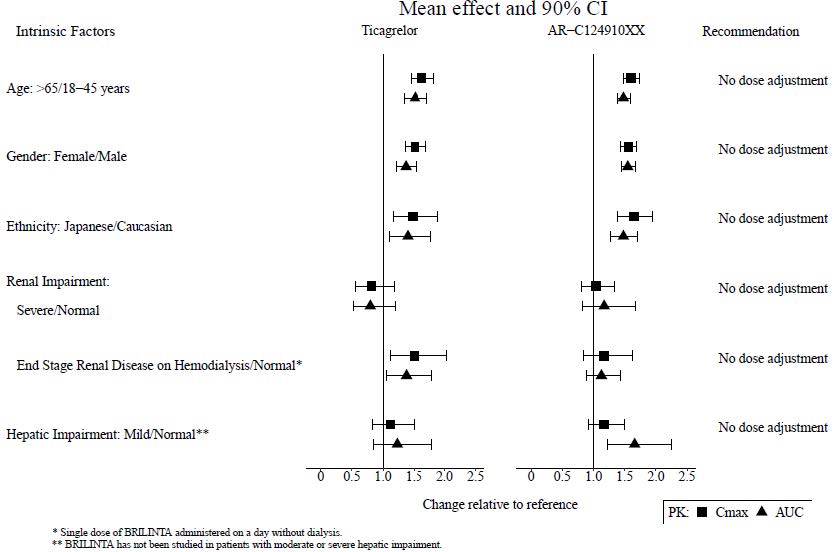
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from case reports with ticagrelor use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse ...
-
10 OVERDOSAGEThere is currently no known treatment to reverse the effects of ticagrelor, and ticagrelor is not dialyzable. Treatment of overdose should follow local standard medical practice. Bleeding is the ...
-
11 DESCRIPTIONTicagrelor tablets contain ticagrelor, a cyclopentyltriazolopyrimidine, inhibitor of platelet activation and aggregation mediated by the P2Y - 12 ADP-receptor. Chemically it is (1 ...
-
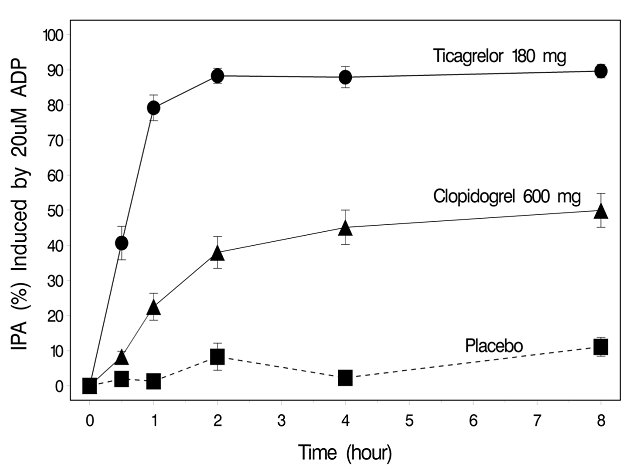
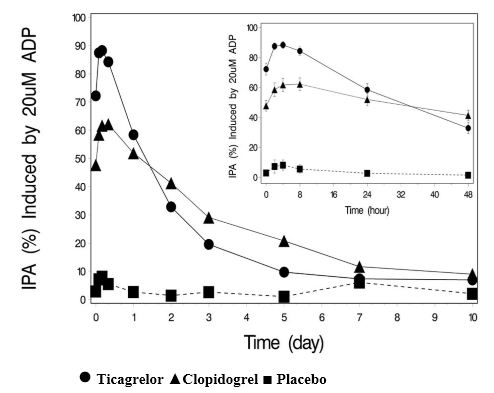
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ticagrelor and its major metabolite reversibly interact with the platelet P2Y - 12 ADP-receptor to prevent signal transduction and platelet activation ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Ticagrelor was not carcinogenic in the mouse at doses up to 250 mg/kg/day or in the male rat at doses up to 120 ...
-
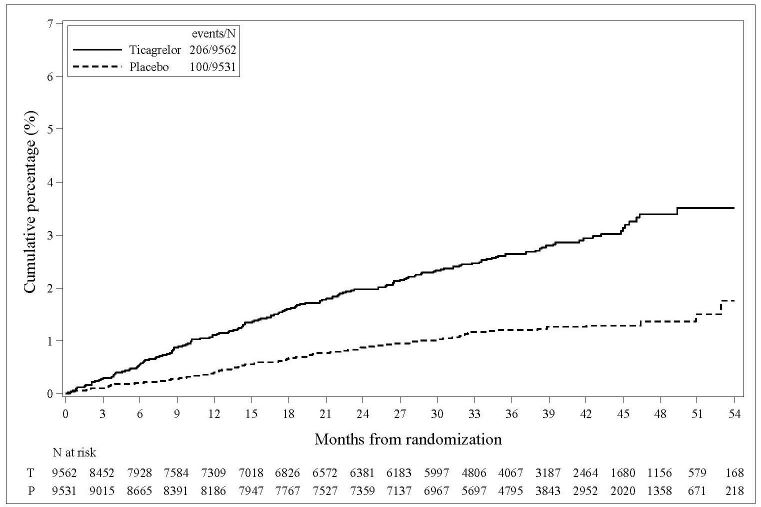
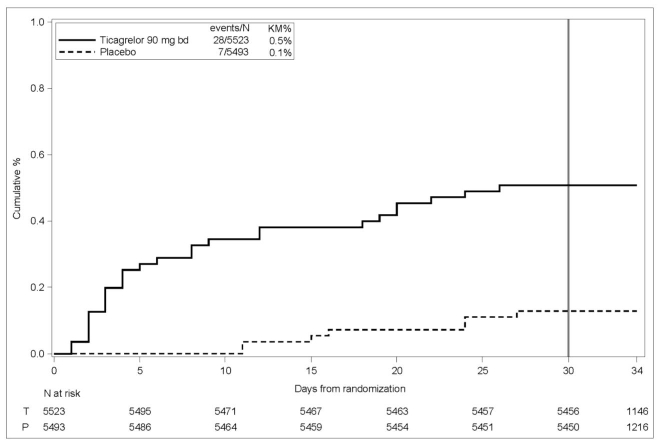
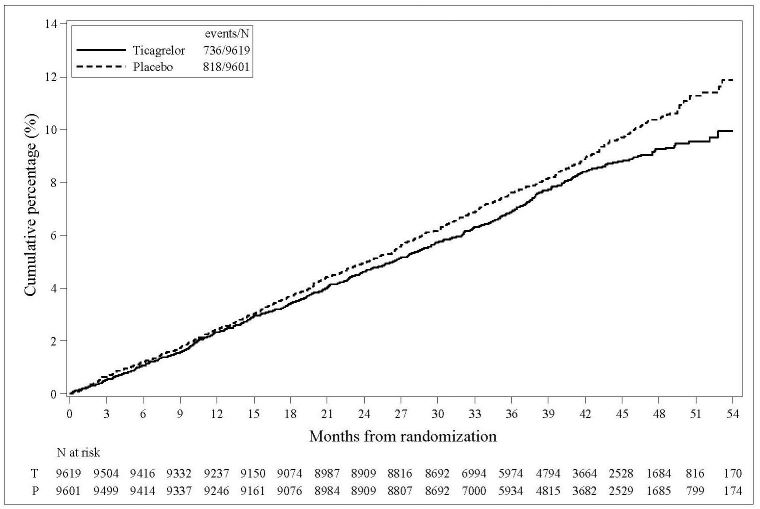
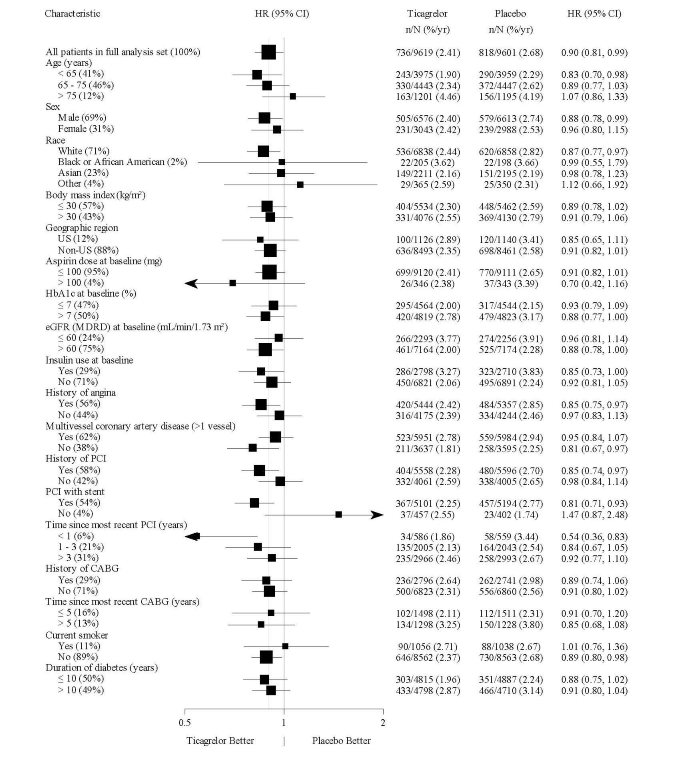
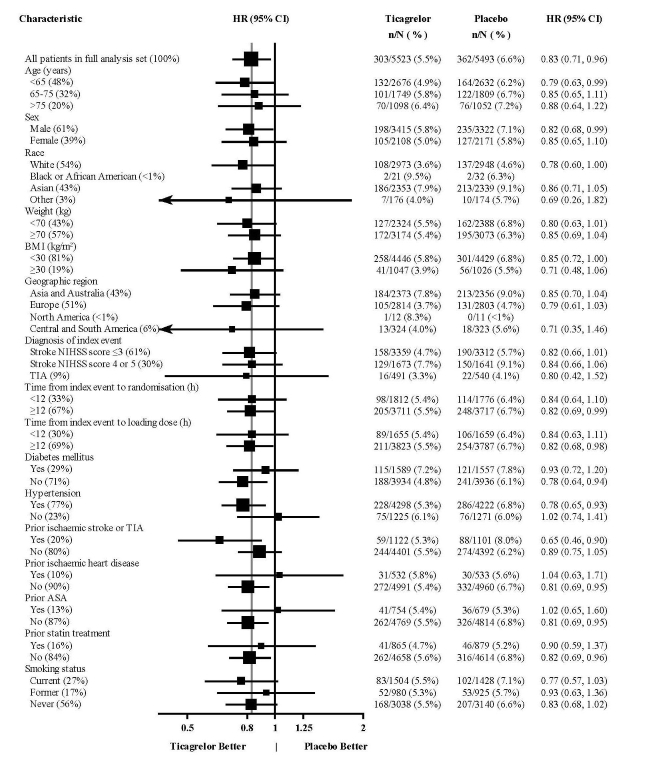
14 CLINICAL STUDIES14.2 Coronary Artery Disease but No Prior Stroke or Myocardial Infarction - THEMIS - The THEMIS study (NCT01991795) was a double-blind, parallel group, study in which 19,220 patients with CAD ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTicagrelor tablets 90 mg are supplied as a round, biconvex, yellow, film-coated tablet debossed with “HU” on one side and “90” on the other side. Bottles of 60 NDC 42658-115-03 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide) Advise patients daily doses of aspirin should not exceed 100 mg and to avoid taking any other medications that ...
-
MEDICATION GUIDETICAGRELOR (tye-KA-grel-or) Tablets - What is the most important information I should know about ticagrelor tablets? Ticagrelor tablets are used to lower your chance of having, or dying from, a ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELTicagrelor Tablets 90 mg/500 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information