Label: GOPATCH HANGOVERRELIEF- calendula officinalis, chamomilla, sepia, tabacum, cocculus indicus, nux vomica patch
- NDC Code(s): 70105-006-05
- Packager: Centered Enterprises, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
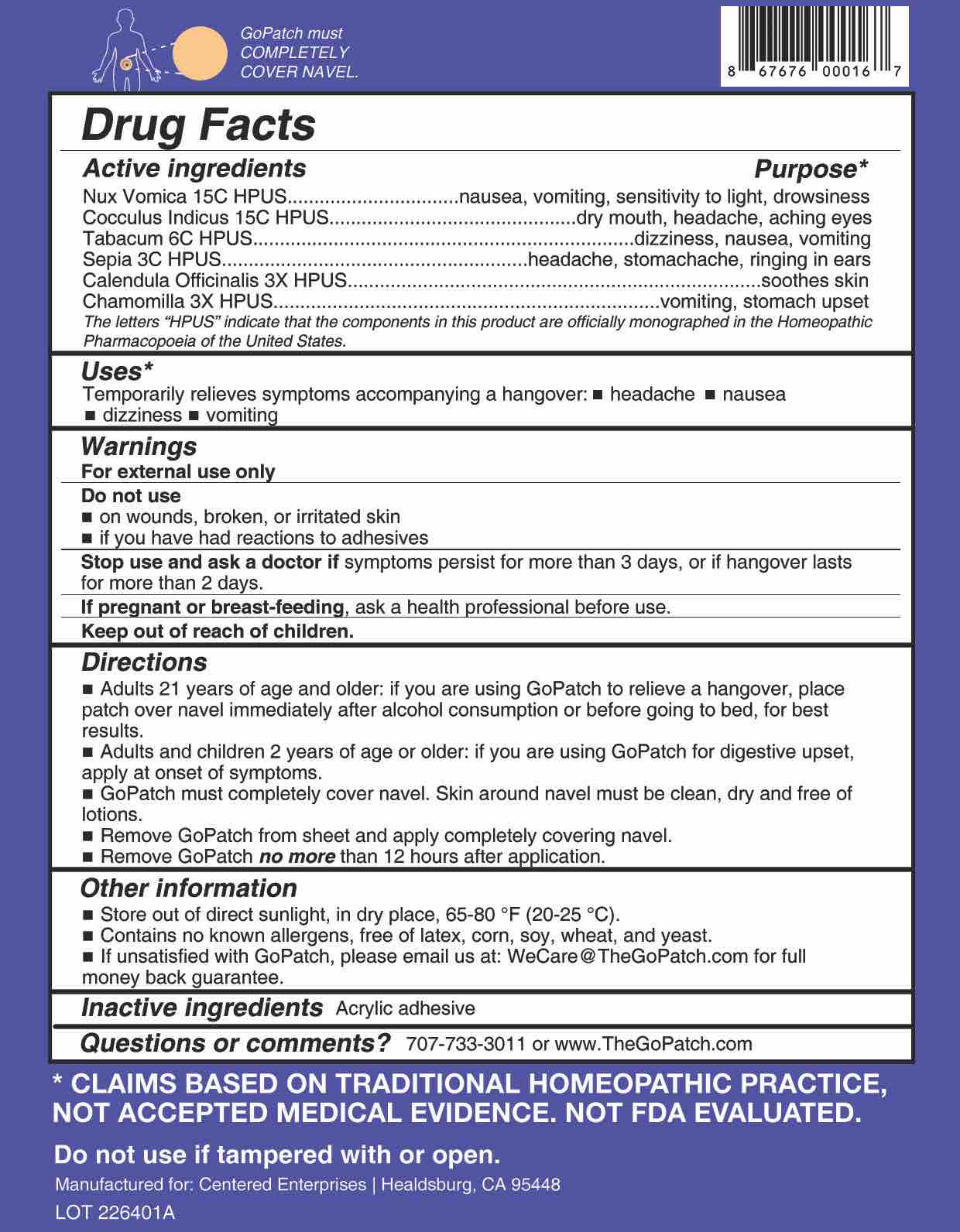
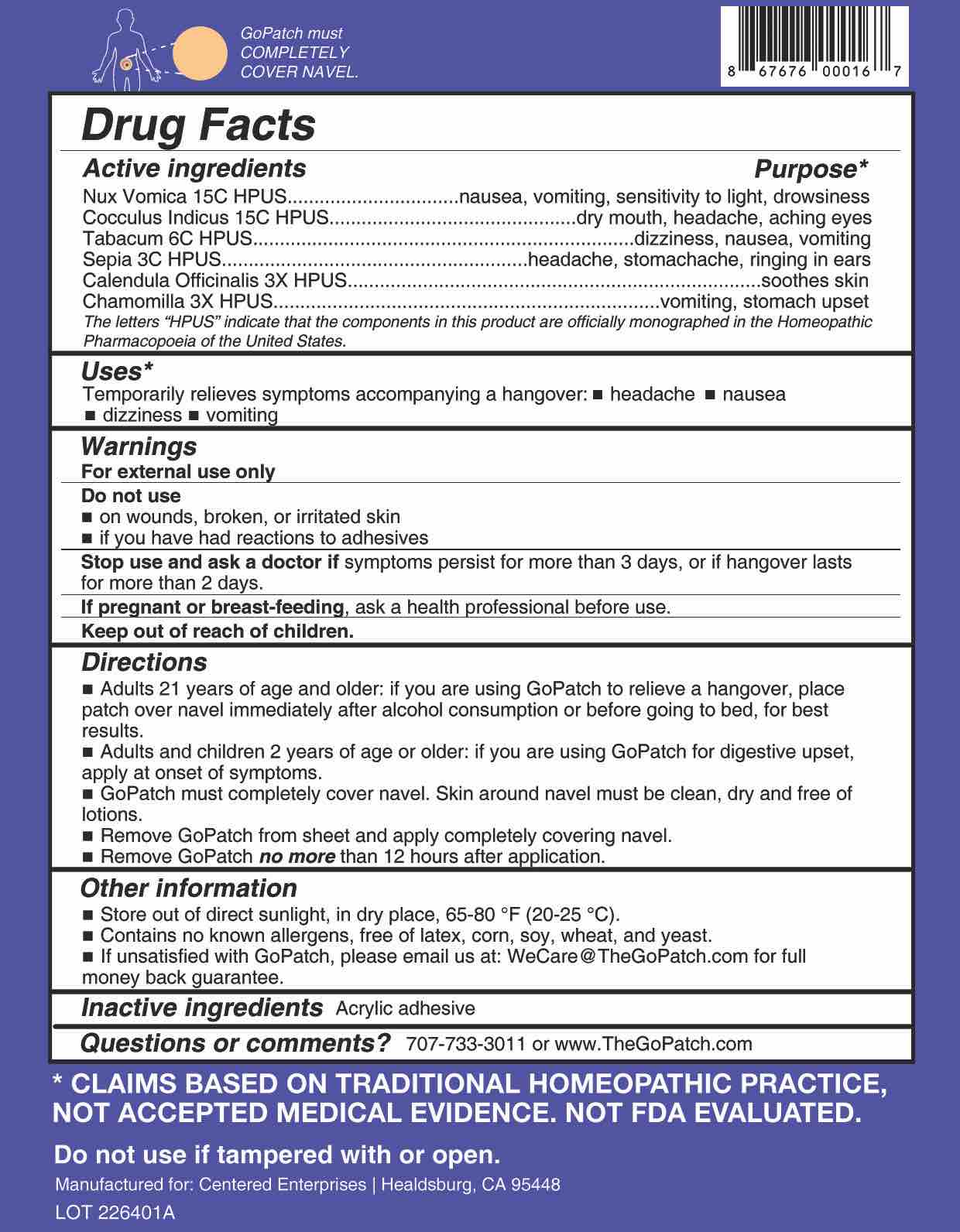
Active ingredients Purpose* Nux Vomica 15C HPUS nausea, vomiting, sensitivity to light, drowsiness
Cocculus Indicus 15C HPUS
dry mouth, headache, aching eyes Tabacum 6C HPUS dizziness, nausea, vomiting Sepia 3C HPUS headache, stomachache, ringing in ears Calendula Officinalis 3X HPUS
soothes skin Chamomilla 3X HPUS vomitting, stomach upset The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
- PURPOSE
- Uses
- WARNINGS
- Do Not Use
- Stop Use
- If pregnant or breast-feeding
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Directions
■ Adults 21 years of age or older: if you are using GoPatch to relieve a hangover, place patch over navel immediately after alcohol consumption or before going to bed, for best results.
■ Adults and children 2 years of age and older: If you are using GoPatch for digestive upset, apply at onset of symptoms.■ GoPatch must completely cover navel. Skin around navel must be clean, dry, and free of lotions.
■ Remove GoPatch from sheet and apply completely covering navel.
■ Remove GoPatch no more than 12 hours after application.
- OTHER SAFETY INFORMATION
- Questions?
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GOPATCH HANGOVERRELIEF
calendula officinalis, chamomilla, sepia, tabacum, cocculus indicus, nux vomica patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70105-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 3 [hp_X] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 3 [hp_X] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 3 [hp_C] TOBACCO LEAF (UNII: 6YR2608RSU) (TOBACCO LEAF - UNII:6YR2608RSU) TOBACCO LEAF 6 [hp_C] ANAMIRTA COCCULUS SEED (UNII: 810258W28U) (ANAMIRTA COCCULUS SEED - UNII:810258W28U) ANAMIRTA COCCULUS SEED 15 [hp_C] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 15 [hp_C] Inactive Ingredients Ingredient Name Strength POLYVINYL ACETATE (UNII: 32K497ZK2U) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70105-006-05 6 in 1 BOX; Type 0: Not a Combination Product 06/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/2021 Labeler - Centered Enterprises, LLC (079240957)