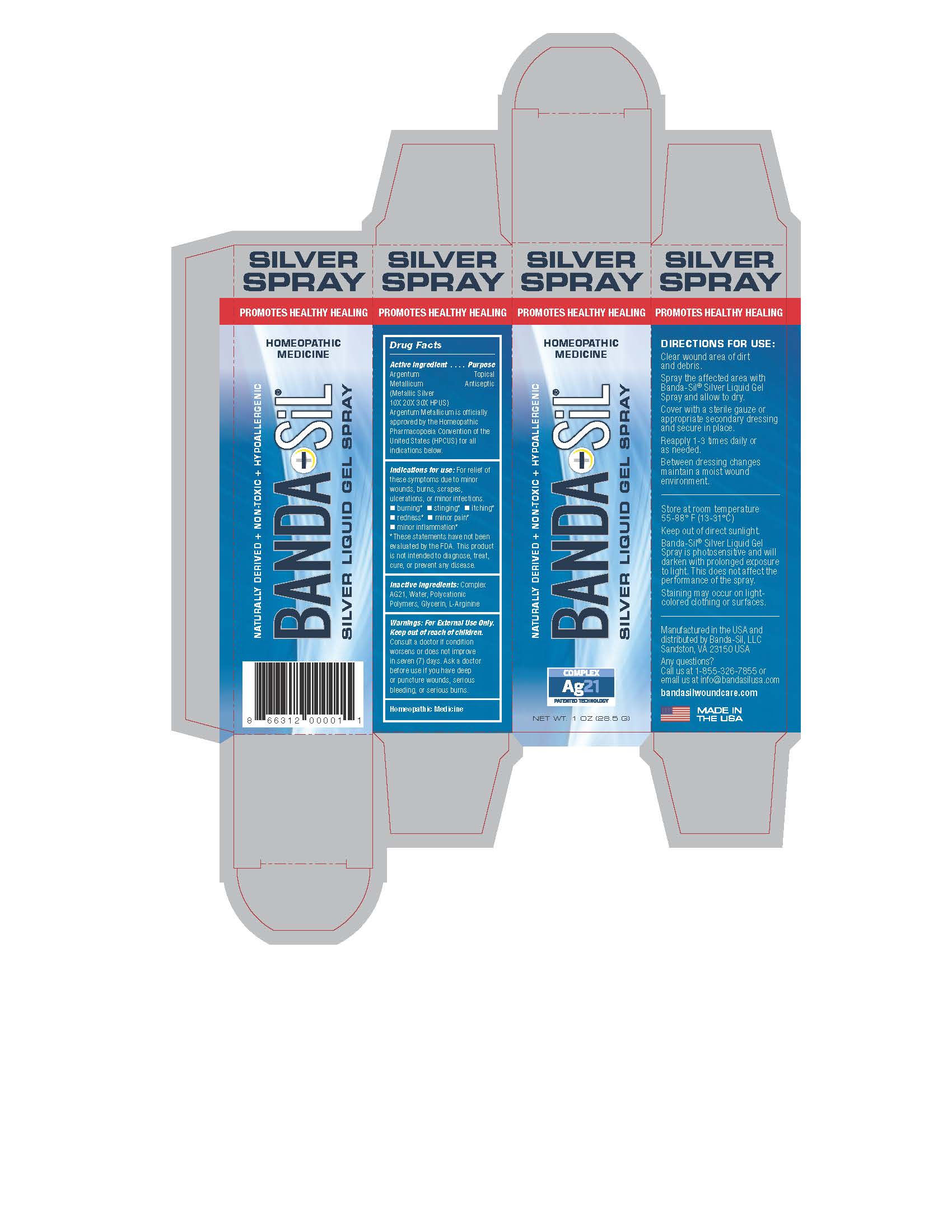
Label: BANDA-SIL LIQUID GEL- argentum metallicum gel
- NDC Code(s): 72363-005-03
- Packager: AG Essence Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DOSAGE & ADMINISTRATION
Directions For Use:
Clear wound area of dirt and debris.
Spray the affected area with Banda-Sil Silver Liquid Gel Spray and allow to dry.
Cover with a sterile gauze or appropriate secondary dressing and secure in place.
Reapply 1-3 times daily or as needed.
Between dressing changes maintain a moist wound environment.
- WARNINGS
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- ACTIVE INGREDIENT
- Banda-Sil Spray Gel Box
-
INGREDIENTS AND APPEARANCE
BANDA-SIL LIQUID GEL
argentum metallicum gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72363-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 1 g in 28.5 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 27.13 g in 28.5 g GLYCERIN (UNII: PDC6A3C0OX) 0.285 g in 28.5 g ARGININE (UNII: 94ZLA3W45F) 0.085 g in 28.5 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72363-005-03 1 in 1 BOX 06/25/2021 1 28.5 g in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/25/2021 Labeler - AG Essence Inc (068562165) Establishment Name Address ID/FEI Business Operations AG Essence Inc 068562165 manufacture(72363-005)