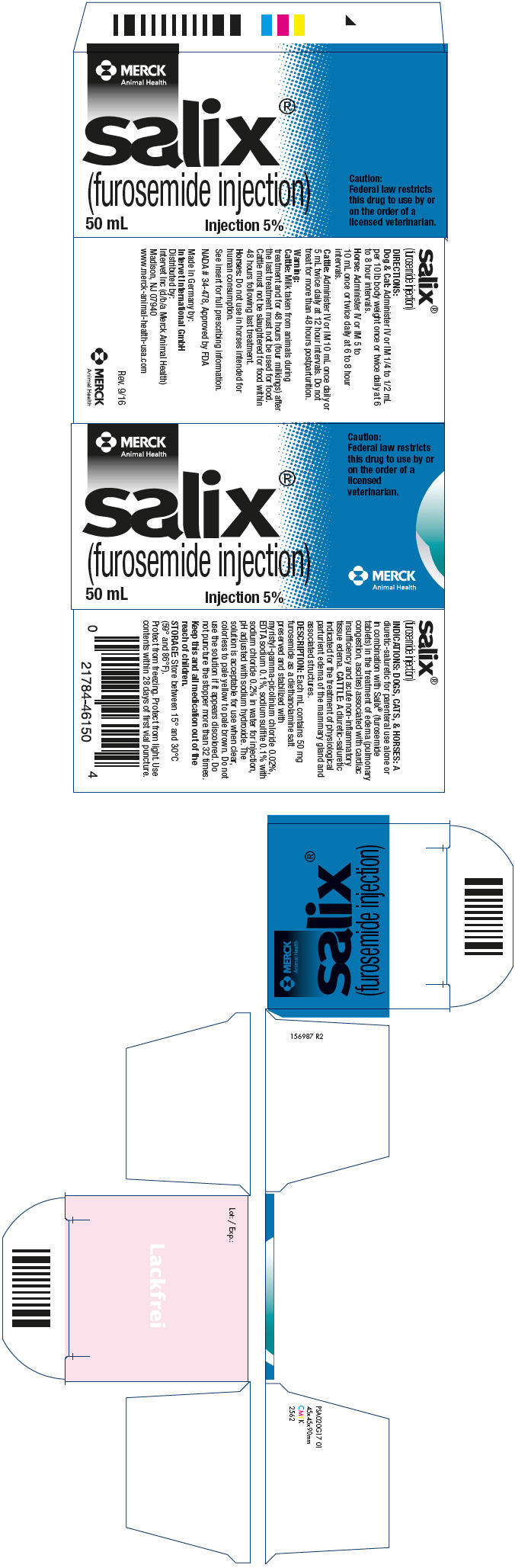
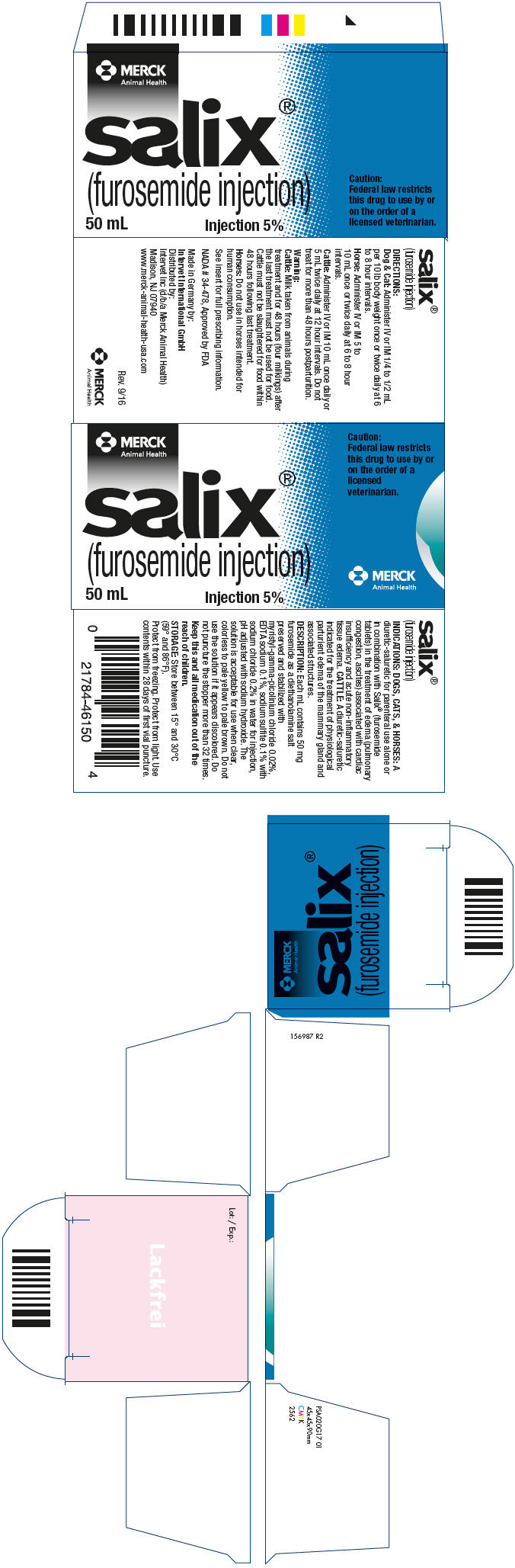
Label: SALIX- furosemide injection
- NDC Code(s): 57926-465-01
- Packager: Merck Sharp & Dohme Corp.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Salix® (furosemide injection) is a chemically distinct diuretic and saluretic pharmacodynamically characterized by the following:
- 1)
- A high degree of efficacy, low-inherent toxicity and a high therapeutic index.
- 2)
- A rapid onset of action and of comparatively short duration.1,2
- 3)
- A pharmacological action in the functional area of the nephron, i.e., proximal and distal tubules and the ascending limb of the loop of Henle.2,3,4
- 4)
- A dose-response relationship and a ratio of minimum to maximum effective dose range greater than tenfold.1,2
- 5)
- It may be administered orally or parenterally. It is readily absorbed from the intestinal tract and well tolerated.
The intravenous route produces the most rapid diuretic response.
The CAS Registry Number is 54-31-9.
Salix®, a diuretic, is an anthranilic acid derivative with the following structural formula:
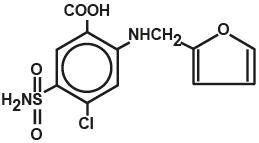
Generic name: Furosemide (except in United Kingdom-furosemide). Chemical name: 4-chloro-N-furfuryl-5-sulfamoylanthranilic acid.
-
ACTIONS
The therapeutic efficacy of Salix® is from the activity of the intact and unaltered molecule throughout the nephron, inhibiting the reabsorption of sodium not only in the proximal and distal tubule but also in the ascending limb of the loop of Henle. The prompt onset of action is a result of the drug's rapid absorption and a poor lipid solubility. The low lipid solubility and a rapid renal excretion minimize the possibility of its accumulation in tissues and organs or crystalluria. Salix® has no inhibitory effect on carbonic anhydrase or aldosterone activity in the distal tubule. The drug possesses diuretic activity either in presence of acidosis or alkalosis.1,2,3,4,5,6,7
-
INDICATIONS
Dogs, Cats & Horses:
Salix® is an effective diuretic possessing a wide therapeutic range. Pharmacologically it promotes the rapid removal of abnormally retained extracellular fluids. The rationale for the efficacious use of diuretic therapy is determined by the clinical pathology producing the edema. Salix® is indicated for the treatment of edema, (pulmonary congestion, ascites) associated with cardiac insufficiency and acute noninflammatory tissue edema.
The continued use of heart stimulants, such as digitalis or its glycosides is indicated in cases of edema involving cardiac insufficiency.
-
CONTRAINDICATIONS - PRECAUTIONS
Salix® is a highly effective diuretic-saluretic which if given in excessive amounts may result in dehydration and electrolyte imbalance. Therefore, the dosage and schedule may have to be adjusted to the patient's needs. The animal should be observed for early signs of electrolyte imbalance, and corrective measures administered. Early signs of electrolyte imbalance are: increased thirst, lethargy, drowsiness or restlessness, fatigue, oliguria, gastro-intestinal disturbances and tachycardia. Special attention should be given to potassium levels. Salix® may lower serum calcium levels and cause tetany in rare cases of animals having an existing hypocalcemic tendency.10,11,12,13,14
Although diabetes mellitus is a rarely reported disease in animals, active or latent diabetes mellitus may on rare occasions be exacerbated by Salix®. While it has not been reported in animals the use of high doses of salicylates, as in rheumatic diseases, in conjunction with Salix® may result in salicylate toxicity because of competition for renal excretory sites.
Transient loss of auditory capacity has been experimentally produced in cats following intravenous injection of excessive doses of Salix® at a very rapid rate.15,16,17
Electrolyte balance should be monitored prior to surgery in patients receiving Salix®. Imbalances must be corrected by administration of suitable fluid therapy.
Salix® is contraindicated in anuria. Therapy should be discontinued in cases of progressive renal disease if increasing azotemia and oliguria occur during the treatment. Sudden alterations of fluid and electrolyte imbalance in an animal with cirrhosis may precipitate hepatic coma, therefore observation during period of therapy is necessary. In hepatic coma and in states of electrolyte depletion, therapy should not be instituted until the basic condition is improved or corrected. Potassium supplementation may be necessary in cases routinely treated with potassium-depleting steroids.
-
WARNINGS
Salix® is a highly effective diuretic and if given in excessive amounts as with any diuretic may lead to excessive diuresis which could result in electrolyte imbalance, dehydration and reduction of plasma volume enhancing the risk of circulatory collapse, thrombosis, and embolism. Therefore, the animal should be observed for early signs of fluid depletion with electrolyte imbalance, and corrective measures administered. Excessive loss of potassium in patients receiving digitalis or its glycosides may precipitate digitalis toxicity. Caution should be exercised in animals administered potassium-depleting steroids.
It is important to correct potassium deficiency with dietary supplementation. Caution should be exercised in prescribing enteric-coated potassium tablets.
There have been several reports in human literature, published and unpublished, concerning non-specific small-bowel lesions consisting of stenosis, with or without ulceration, associated with the administration of enteric-coated thiazides with potassium salts. These lesions may occur with enteric-coated potassium tablets alone or when they are used with nonenteric-coated thiazides, or certain other oral diuretics. These small-bowel lesions may have caused obstruction, hemorrhage, and perforation. Surgery was frequently required, and deaths have occurred. Available information tends to implicate enteric-coated potassium salts, although lesions of this type also occur spontaneously. Therefore, coated potassium-containing formulations should be administered only when indicated and should be discontinued immediately if abdominal pain, distention, nausea, vomiting, or gastro-intestinal bleeding occurs.
Human patients with known sulfonamide sensitivity may show allergic reactions to Salix®; however, these reactions have not been reported in animals.
Sulfonamide diuretics have been reported to decrease arterial responsiveness to pressor amines and to enhance the effect of tubocurarine. Caution should be exercised in administering curare or its derivatives to patients undergoing therapy with Salix® and it is advisable to discontinue Salix® for one day prior to any elective surgery.
- WARNING
-
DOSAGE AND ADMINISTRATION
The usual dosage of Salix® is 1 to 2 mg/lb. body weight (approximately 2.5 to 5 mg/kg). The lower dosage is suggested for cats. Administer once or twice daily at 6 to 8 hour intervals either orally, intravenously, or intramuscularly. A prompt diuresis usually ensues from the initial treatment. Diuresis may be initiated by the parenteral administration of Salix® Injection and then maintained by oral administration.
The dosage should be adjusted to the individual's response. In severe edematous or refractory cases, the dose may be doubled or increased by increments of 1 mg per pound body weight. The established effective dose should be administered once or twice daily. The daily schedule of administration can be timed to control the period of micturition for the convenience of the client or veterinarian. Mobilization of the edema may be most efficiently and safely accomplished by utilizing an intermittent daily dosage schedule, i.e., every other day or 2 to 4 consecutive days weekly.
Diuretic therapy should be discontinued after reduction of the edema, or maintained after determining a carefully programmed dosage schedule to prevent recurrence of edema. For long-term treatment, the dose can generally be lowered after the edema has once been reduced. Re-examination and consultations with client will enhance the establishment of a satisfactorily programmed dosage schedule. Clinical examination and serum BUN, CO2 and electrolyte determinations should be performed during the early period of therapy and periodically thereafter, especially in refractory cases. Abnormalities should be corrected or the drug temporarily withdrawn.
DOSAGE:
The solution is acceptable for use when clear, colorless to pale yellow to pale brown. Do not use this solution if it appears discolored. Do not puncture the stopper more than 32 times.
DOG AND CAT
Administer intramuscularly or intravenously 1/4 to 1/2 mL per 10 pounds body weight.
Administer once or twice daily, permitting a 6 to 8 hour interval between treatments. In refractory or severe edematous cases, the dosage may be doubled or increased by increments of 1 mg per pound body weight as recommended in preceding paragraphs, "Dosage and Administration".
HORSE
The individual dose is 250 mg to 500 mg (5 to 10 mL) administered intramuscularly or intravenously once or twice daily at 6 to 8 hour intervals until desired results are achieved. The veterinarian should evaluate the degree of edema present and adjust dosage schedule accordingly. Do not use in horses intended for human consumption.
CATTLE
The individual dose administered intramuscularly or intravenously is 500 mg (10 mL) once daily or 250 mg (5 mL) twice daily at 12 hour intervals. Treatment not to exceed 48 hours postparturition.
Milk taken from animals during treatment and for 48 hours (four milkings) after the last treatment must not be used for food. Cattle must not be slaughtered for food within 48 hours following last treatment.
-
HOW SUPPLIED/STORAGE AND HANDLING
Salix® (furosemide injection) 5% Each mL contains: 50 mg furosemide as a diethanolamine salt preserved and stabilized with myristyl-gamma-picolinium chloride 0.02%, EDTA sodium 0.1%, sodium sulfite 0.1% with sodium chloride 0.2% in distilled water, pH adjusted with sodium hydroxide.
Available in 50 mL multidose vials.
-
TOXICOLOGY
Acute Toxicity:
The following table illustrates low acute toxicity of Salix® in three different species.
(Two values indicate two different studies.)
LD50 of Salix® in mg/kg body weight SPECIES INTRAVENOUS *NOTE: The lower value for the rat oral LD50 was obtained in a group of fasted animals; the higher figure is from a study performed in fed rats. Mouse 308 Rat 680 Dog >300 and >464 Toxic doses lead to convulsions, ataxia, paralysis and collapse. Animals surviving toxic dosages may become dehydrated and depleted of electrolytes due to the massive diuresis and saluresis.
Chronic Toxicity:
Chronic toxicity studies with Salix® were done in a one-year study in rats and dogs. In a one-year study in rats, renal tubular degeneration occurred with all doses higher than 50 mg/kg. A six-month study in dogs revealed calcification and scarring of the renal parenchyma at all doses above 10 mg/kg.
Reproductive Studies:
Reproductive studies were conducted in mice, rats and rabbits. Only in rabbits administered high doses (equivalent to 10 to 25 times the recommended average dose of 2 mg/kg for dogs, cats, horses, and cattle) of furosemide during the second trimester period did unexplained maternal deaths and abortions occur. The administration of Salix® is not recommended during the second trimester of pregnancy.
-
REFERENCES
- Timmerman, R.J.; Springman, F.R., and Thoms, R.K.: Evaluation of Furosemide, a New Diuretic Agent. Current Therapeutic Research 6(2):88-94, February 1964.
- Muschaweck, R., and Hajdu, P.: Die salidiuretische Wirksamkeit der Chlor-N-(2-furylmethyl)-sulfamyl-anthranilsaure. Arzneimittel-Forschung 14:44-47, 1964. (The Saluretic Action of 4-Chloro-N-(2-furylmethyl)-5-sulfamyl-anthranilic acid).
- Suki, W.; Rector, Jr., F.C., and Seldin, D.W.: The Site of Action of Furosemide and Other Sulfonamide Diuretics in the Dog. Journal of Clinical Investigation 44(9): 1458-1469,1965.
- Deetjen, P.: Mikropunktionsunter-suchungen zur Wirkung von Furosemid. Pflugers Archiv fuer die Gesamte Physiologie 284:184-190, 1965 (Micropuncture Studies of the Action of Furosemide).
- Berman, L.B., and Ebrahimi, A.: Experiences with Furosemide in Renal Disease. Proceedings of the Society for Experimental Biology and Medicine 188:333-336, February 1965.
- Schmidt, H.A.E.: "Animal Experiments with S35 Tagged Lasix® in Canine and Ovine." Radio-chemical Pharmacological Laboratory, Farbwerke Hoechst, Frankfurt, West Germany.
- Haussler, A., and Hajdu, P.: "Methods Biological Identification and Results of Studies on Absorption, Elimination and Metabolism." Research Laboratories, Farbwerke Hoechst, Frankfurt, West Germany.
- Wilson, A.F., and Simmons, D.H.: Diuretic Action in Hypochloremic Dogs. Clinical Research 14(1):158, January 1966.
- Hook, J.B., and Williamson, H.E.: Influence of Probenecid and Alterations in Acid-Base Balance of the Saluretic Activity of Furosemide. Journal of Pharmacology and Experimental Therapeutics: 149(3):404-408, 1965.
- Antoniou, L.D.; Eisner, G.M.; Slotkoff, L.M. and Lilienfield, L.S.: Sodium and Calcium Transport in the Kidney. Clinical Research 15(4):476, December 1967.
- Duarte, C.G.: Effects of Furosemide (F) and Ethacrynic Acid (ETA) on the Renal Clearance of Phosphate (Cp), Ultrafilterable Calcium (CUfCa) and Magnesium (CUfMg). Clinical Research 15(2):357, April 1967.
- Duarte, C.G.: Effects of Ethacrynic Acid and Furosemide on Urinary Calcium, Phosphate and Magnesium. Metabolism 17:867-876, October 1968.
- Nielsen, S.P.; Andersen, O., and Steven, K.E.: Magnesium and Calcium Metabolism during Prolonged Furosemide (Lasix®) Administration to Normal Rats. Acta Pharmacol. et Toxicol. 1969, 27:469-479.
- Reimold, E.W.: The effect of Furosemide on Hypercalcemia Due to Dihydrotachysterol. Metabolism 21(7), July 1972.
- Brown, R.D., and McElwee, Jr., T.W.: Effects of Intra-Arterially and Intravenously Administered Ethacrynic Acid and Furosemide on Cochlear N, in Cats. Toxicology and Applied Pharmacology 22: 589-594, 1972.
- Mathog, R.H.; Thomas, W.G., and Hudson, W.R.: Ototoxicity of New and Potent Diuretics. Archives of Otolaryngology 92(l):7-13, July 1970.
- Mathog, R.H., and Matz, G.J.: Ototoxic Effects of Ethacrynic Acid. Annals of Otolaryngology Vol. 81, 1972.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
SALIX
furosemide injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:57926-465 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FUROSEMIDE (UNII: 7LXU5N7ZO5) (FUROSEMIDE - UNII:7LXU5N7ZO5) FUROSEMIDE 50 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57926-465-01 1 in 1 CARTON 1 50 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA034478 09/01/1967 Labeler - Merck Sharp & Dohme Corp. (001317601)