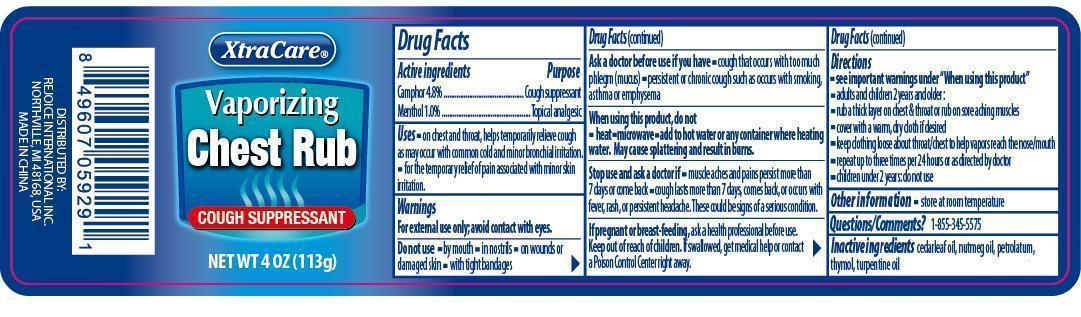
Label: XTRACARE VAPORIZING CHEST RUB- camphor, menthol gel
- NDC Code(s): 57337-051-01
- Packager: Rejoice International
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only; avoid contact with eyes.
Do not use
- by mouth
- in nostrils
- on wounds or damaged skin
- with tight bandages
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma or emphysema
When using this product, do not
- heat
- microwave
- add to hot water or any container where heating water. May cause splattering and result in burns.
Stop use and ask a doctor if
- muscle aches and pains persist more than 7 days or come back
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
-
DOSAGE & ADMINISTRATION
Directions
- see important warnings under "When using this product"
- adults and children 2 years old and older:
- rub a thick layer on chest & throat or rub on sore aching muscles
- cover with a warm, dry cloth if desired
- keep clothing loose about throat chest to help vapors reach the nose/mouth
- repeat up to three times per 24 hours or as directed by doctor
- children under 2 years: do not use
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
XTRACARE VAPORIZING CHEST RUB
camphor, menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57337-051 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 5 g in 113 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 113 g Inactive Ingredients Ingredient Name Strength CEDAR LEAF OIL (UNII: BJ169U4NLG) NUTMEG OIL (UNII: Z1CLM48948) PETROLATUM (UNII: 4T6H12BN9U) THYMOL (UNII: 3J50XA376E) TURPENTINE OIL (UNII: C5H0QJ6V7F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57337-051-01 113 g in 1 BOTTLE; Type 0: Not a Combination Product 01/30/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/30/2014 Labeler - Rejoice International (078741245) Establishment Name Address ID/FEI Business Operations China Ningbo Shangge Cosmetic Technology Corp. 529287434 manufacture(57337-051)