Label: SEVELAMER CARBONATE powder, for suspension
- NDC Code(s): 69452-126-19, 69452-126-60, 69452-127-19, 69452-127-60
- Packager: Bionpharma Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SEVELAMER CARBONATE FOR ORAL SUSPENSION safely and effectively. See full prescribing information for SEVELAMER CARBONATE FOR ORAL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESevelamer carbonate for oral suspension is indicated for the control of serum phosphorus in adults and children 6 years of age and older with chronic kidney disease (CKD) on dialysis.
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information - Starting Dose for AdultPatients Not Taking a Phosphate Binder.The recommended starting dose of sevelamer carbonate for oral suspension is 0.8 to 1.6 g taken ...
-
3 DOSAGE FORMS AND STRENGTHSPowder: 0.8 g and 2.4 g white to yellowish granular powder packaged in an opaque, foil-lined, heat-sealed packets
-
4 CONTRAINDICATIONSSevelamer carbonate is contraindicated in patients with bowel obstruction. Sevelamer carbonate is contraindicated in patients with known hypersensitivity to sevelamer carbonate, sevelamer ...
-
5 WARNINGS AND PRECAUTIONS5.1 Gastrointestinal Adverse Events - Patients with dysphagia, swallowing disorders, severe gastrointestinal (GI) motility disorders, including severe constipation, or major GI tract surgery were ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSThere are no empirical data on avoiding drug interactions between sevelamer carbonate and most concomitant oral drugs. For oral medication where a reduction in the bioavailability of that ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Sevelamer carbonate is not absorbed systemically following oral administration and maternal use is not expected to result in fetal exposure to the drug. Clinical ...
-
10 OVERDOSAGEIn CKD patients on dialysis, the maximum dose studied was 14 grams of sevelamer carbonate and 13 grams of sevelamer hydrochloride. There are no reports of overdosage with sevelamer carbonate or ...
-
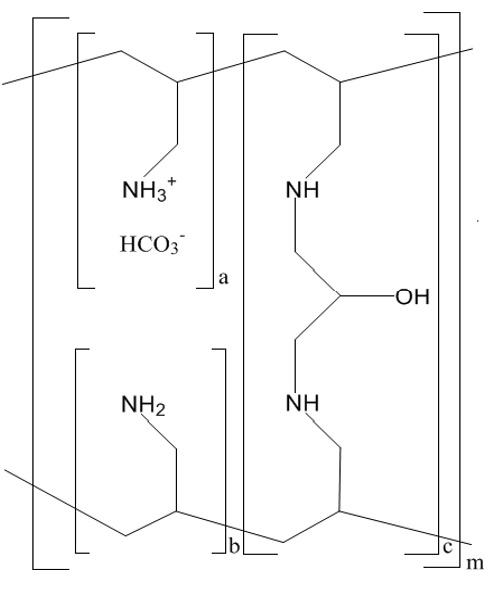
11 DESCRIPTIONThe active ingredient in sevelamer carbonate for oral suspension is sevelamer carbonate, a polymeric amine that binds phosphate and is meant for oral administration. It was developed as a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sevelamer carbonate for oral suspension contains sevelamer carbonate, a non-absorbed phosphate-binding cross-linked polymer, free of metal and calcium. It contains ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Standard lifetime carcinogenicity bioassays were conducted in mice and rats. Rats were given sevelamer hydrochloride by diet at 0.3, 1 ...
-
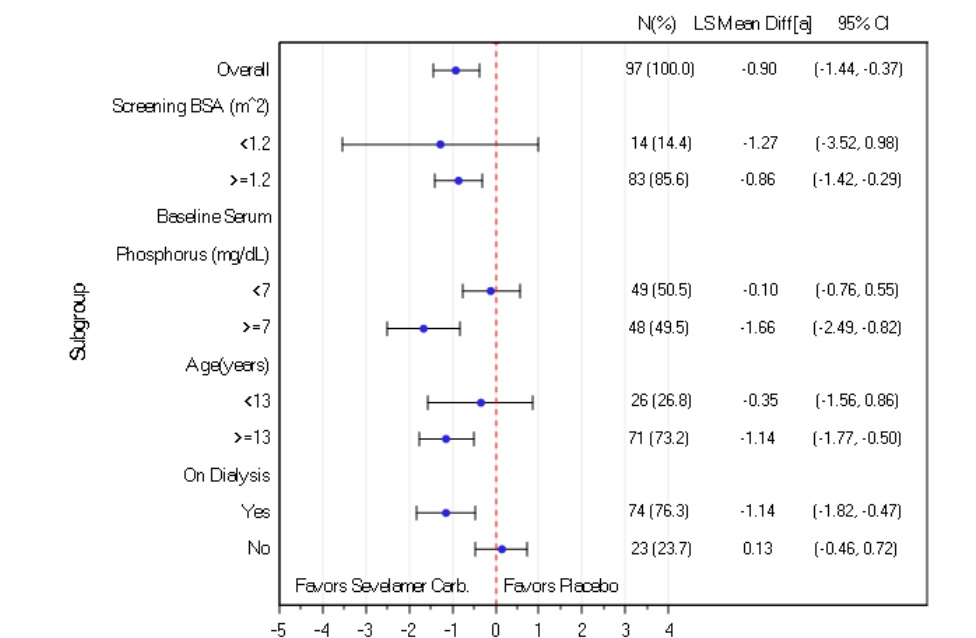
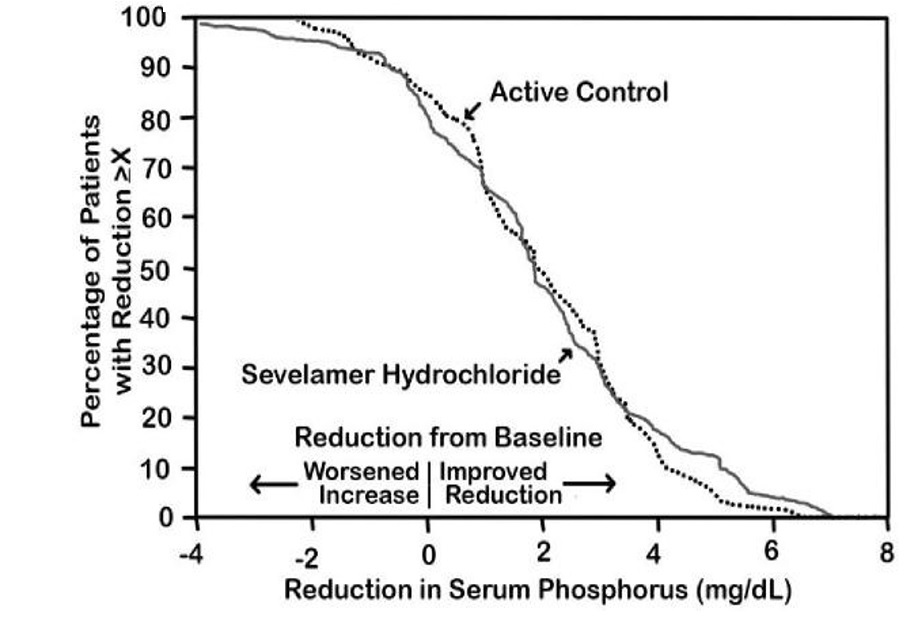
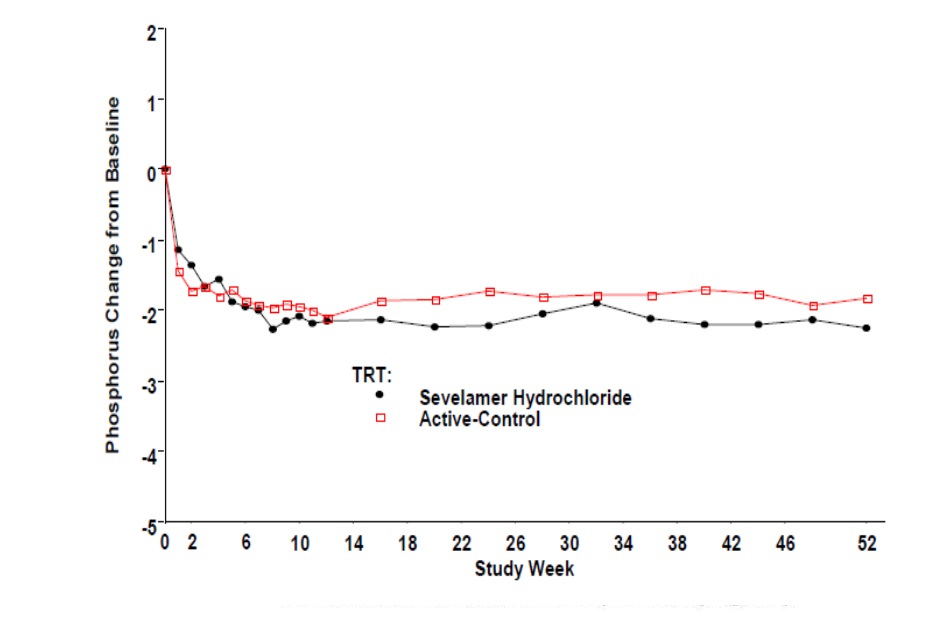
14 CLINICAL STUDIESThe ability of sevelamer to control serum phosphorus in CKD patients on dialysis was predominantly determined from the effects of the hydrochloride salt to bind phosphate. Six clinical trials used ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSevelamer carbonate for oral suspension is supplied as opaque, foil-lined, heat-sealed, packets containing 0.8 g or 2.4 g of sevelamer carbonate on an anhydrous basis. 1 Box (NDC 69452-126-19) of ...
-
17 PATIENT COUNSELING INFORMATIONInform patients to take sevelamer carbonate for oral suspension with meals and adhere to their prescribed diets. For patients using an oral medication where a reduction in the bioavailability of ...
-
Package Label - Principal Display Panel - 0.8 g LabelNDC 69452-126-60 - Sevelamer Carbonate - For Oral Suspension - 0.8 g - Lemon Flavor - Rx only - Front - Back
-
Package Label - Principal Display Panel - 0.8 g Packets, 90 per CartonNDC 69452-126-19 - Sevelamer Carbonate - For Oral Suspension - 0.8 g packets - Lemon Flavor - Rx only - Contains 90 packets
-
Package Label - Principal Display Panel - 2.4 g LabelNDC 69452-127-60 - Sevelamer Carbonate - For Oral Suspension - 2.4 g - Lemon Flavor - Rx only - Front - Back
-
Package Label - Principal Display Panel - 2.4 g Packets, 90 per CartonNDC 69452-127-19 - Sevelamer Carbonate - For Oral Suspension - 2.4 g packets - Lemon Flavor - Rx only - Contains 90 packets
-
INGREDIENTS AND APPEARANCEProduct Information