Label: PHYTONADIONE injection, emulsion
- NDC Code(s): 68083-606-05, 68083-606-25
- Packager: Gland Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING — INTRAVENOUS AND INTRAMUSCULAR USE
Severe reactions, including fatalities, have occurred during and immediately after INTRAVENOUS injection of phytonadione, even when precautions have been taken to dilute the phytonadione and to avoid rapid infusion. Severe reactions, including fatalities, have also been reported following INTRAMUSCULAR administration.
Close
Typically these severe reactions have resembled hypersensitivity or anaphylaxis, including shock and cardiac and/or respiratory arrest. Some patients have exhibited these severe reactions on receiving phytonadione for the first time.
Therefore the INTRAVENOUS and INTRAMUSCULAR routes should be restricted to those situations where the subcutaneous route is not feasible and the serious risk involved is considered justified. -
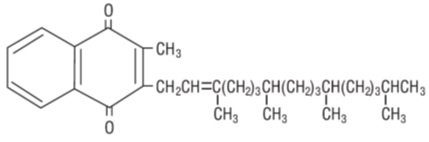
DESCRIPTIONPhytonadione is a vitamin, which is a clear, yellow to amber, viscous, odorless or nearly odorless liquid. It is practically insoluble in water, sparingly soluble in ethanol and miscible with ...
-
CLINICAL PHARMACOLOGYPhytonadione Injectable Emulsion, aqueous dispersion of Phytonadione for parenteral injection, possesses the same type and degree of activity as does naturally-occurring vitamin K, which is ...
-
INDICATIONS AND USAGEPhytonadione Injectable Emulsion is indicated in the following coagulation disorders which are due to faulty formation of factors II, VII, IX and X when caused by vitamin K deficiency or ...
-
CONTRAINDICATIONSHypersensitivity to any component of this medication.
-
WARNINGSBenzyl alcohol as a preservative in Bacteriostatic Sodium Chloride Injection has been associated with toxicity in newborns. Data are unavailable on the toxicity of other preservatives in this ...
-
PRECAUTIONSDrug Interactions - Temporary resistance to prothrombin-depressing anticoagulants may result, especially when larger doses of phytonadione are used. If relatively large doses have been ...
-
ADVERSE REACTIONSDeaths have occurred after intravenous and intramuscular administration. (See Box Warning.) Transient "flushing sensations" and "peculiar" sensations of taste have been observed, as well as ...
-
OVERDOSAGEThe intravenous LD50 of Phytonadione Injectable Emulsion in the mouse is 41.5 and 52 mL/kg for the 0.2% and 1% concentrations, respectively.
-
DOSAGE AND ADMINISTRATIONWhenever possible, Phytonadione Injectable Emulsion, USP should be given by the subcutaneous route (See Box Warning). When intravenous administration is considered unavoidable, the drug should ...
-
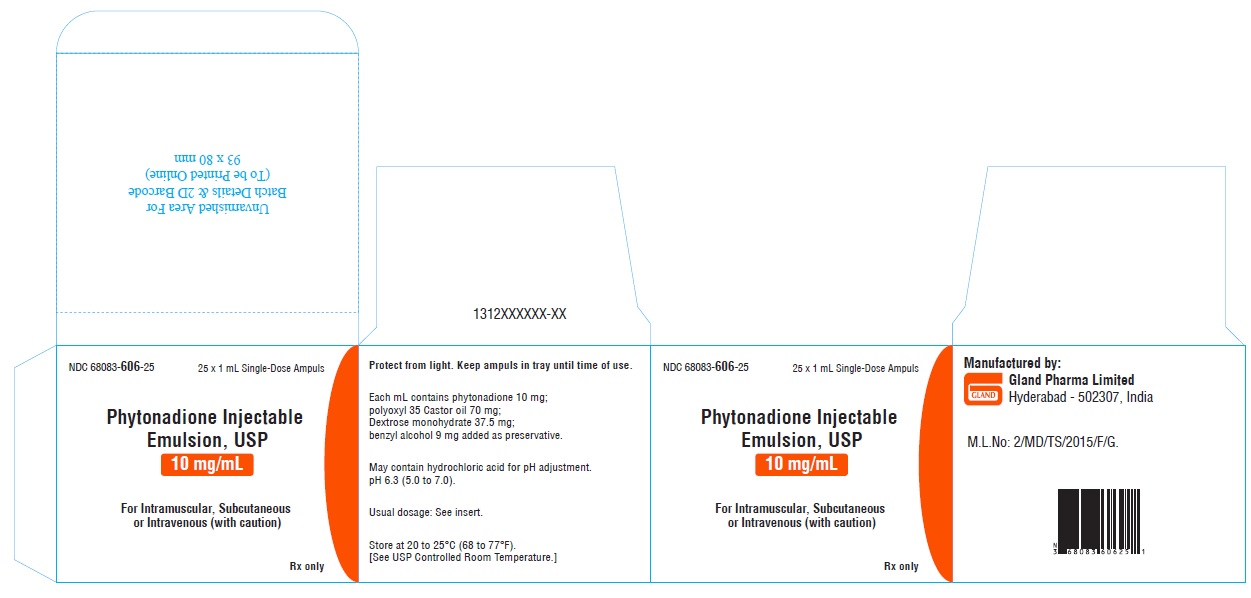
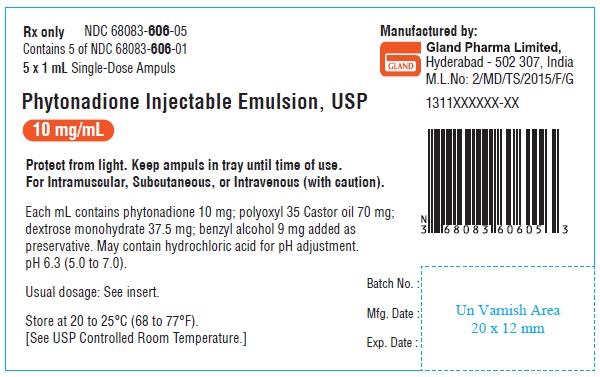
HOW SUPPLIEDPhytonadione Injectable Emulsion, USP is supplied as follows: Unit of Sale - Concentration - NDC 68083-606-25 - 25 ampuls (Bundle of 5 clamcells, each clamcell containing 5 ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELOuter carton Label - NDC 68083-606-25 25 x 1 mL Single-Dose Ampuls - Phytonadione Injectable - Emulsion, USP - 10 mg/mL For Intramuscular, Subcutaneous - or Intravenous (with caution) Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information