Label: SCARLIGHT MD- hydroquinone liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 64269-9902-7, 64269-9902-8 - Packager: Scarguard Labs, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 29, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
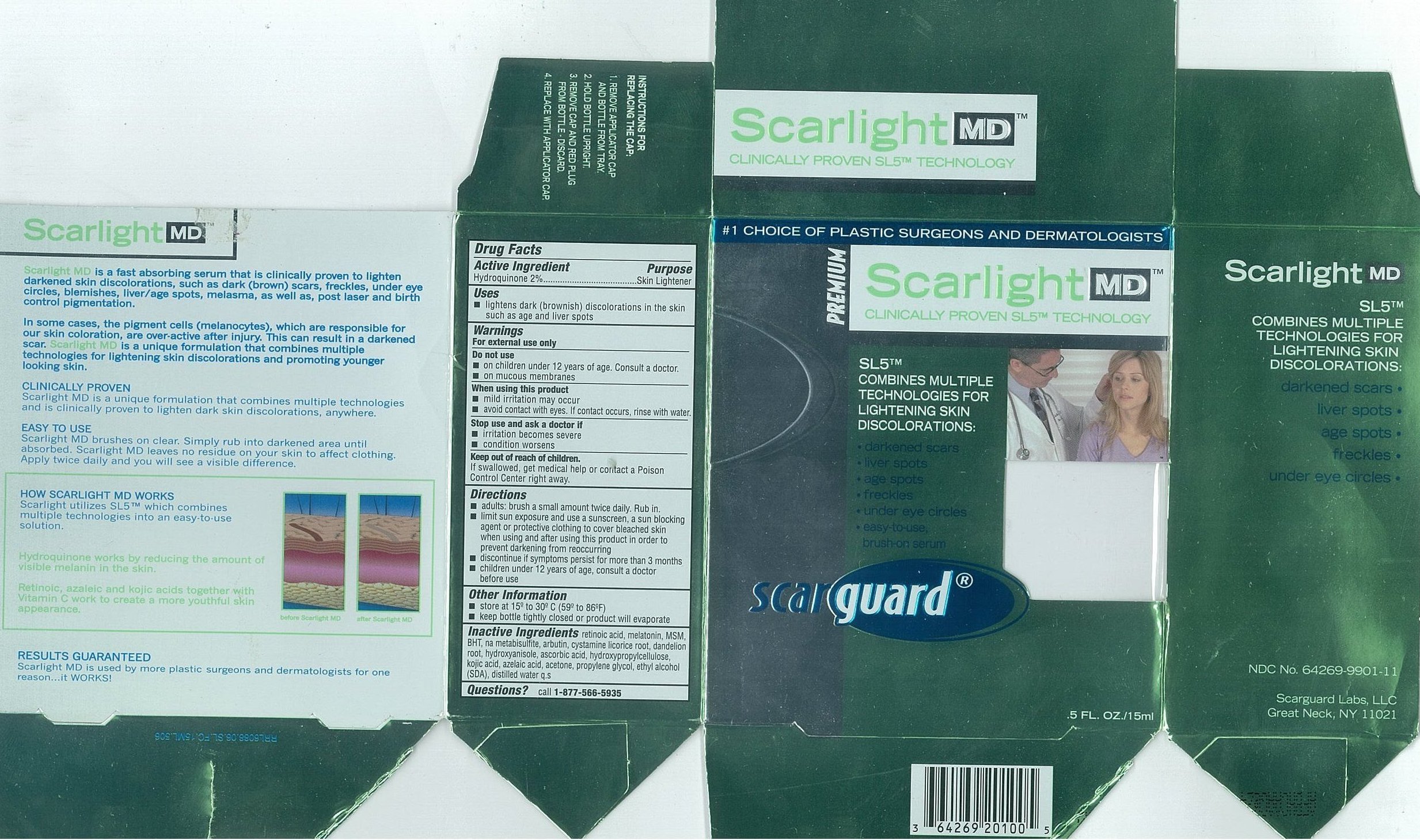
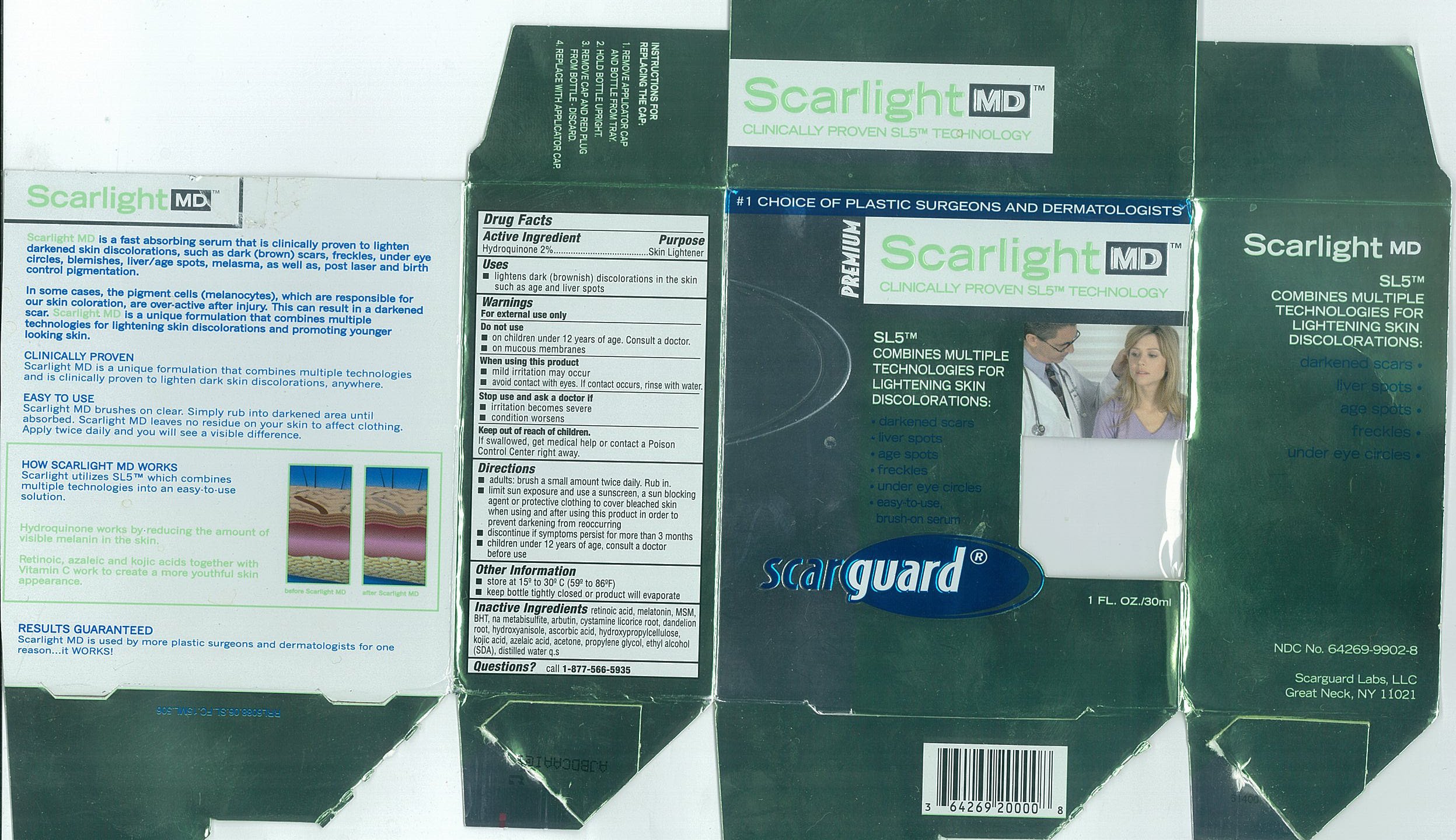
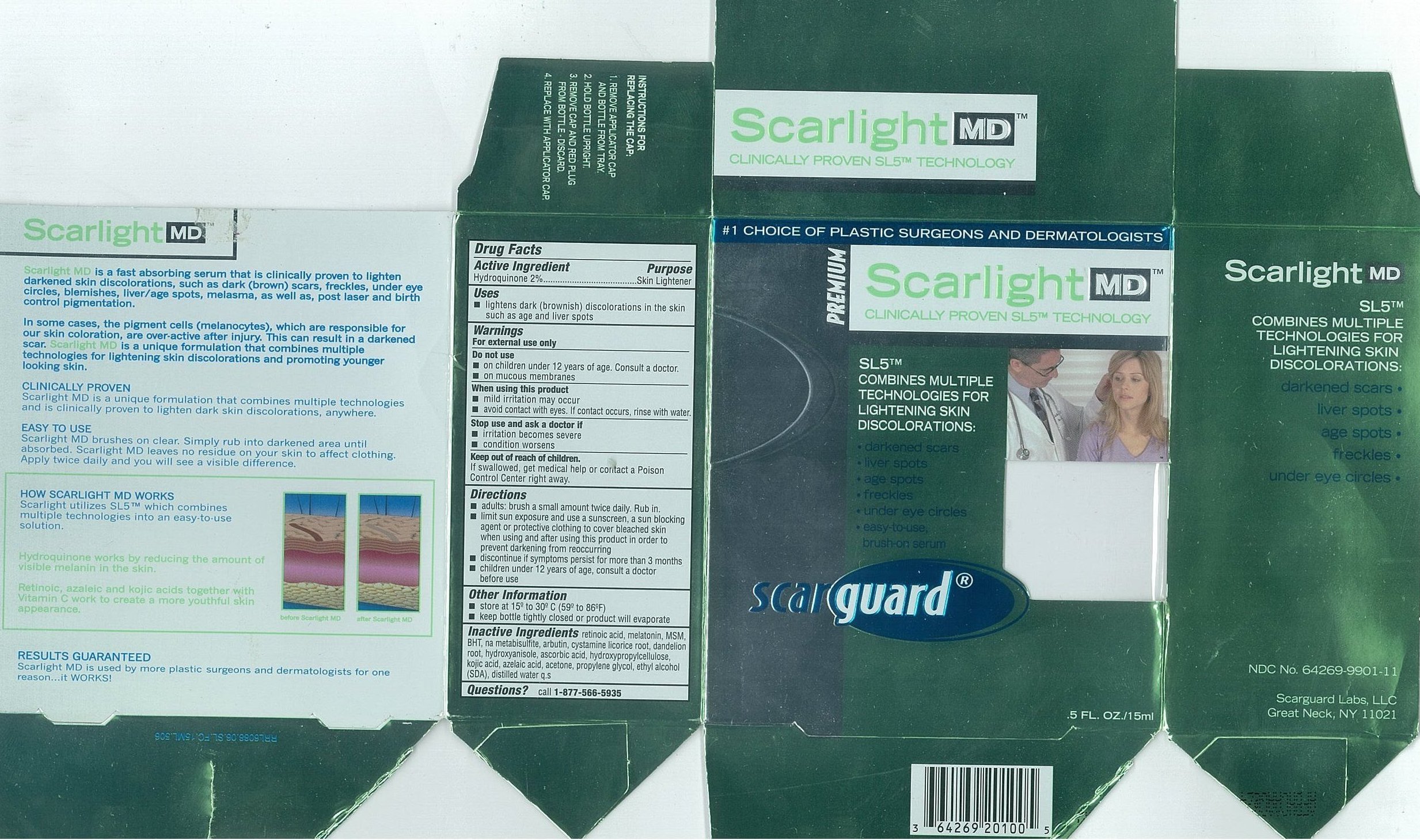
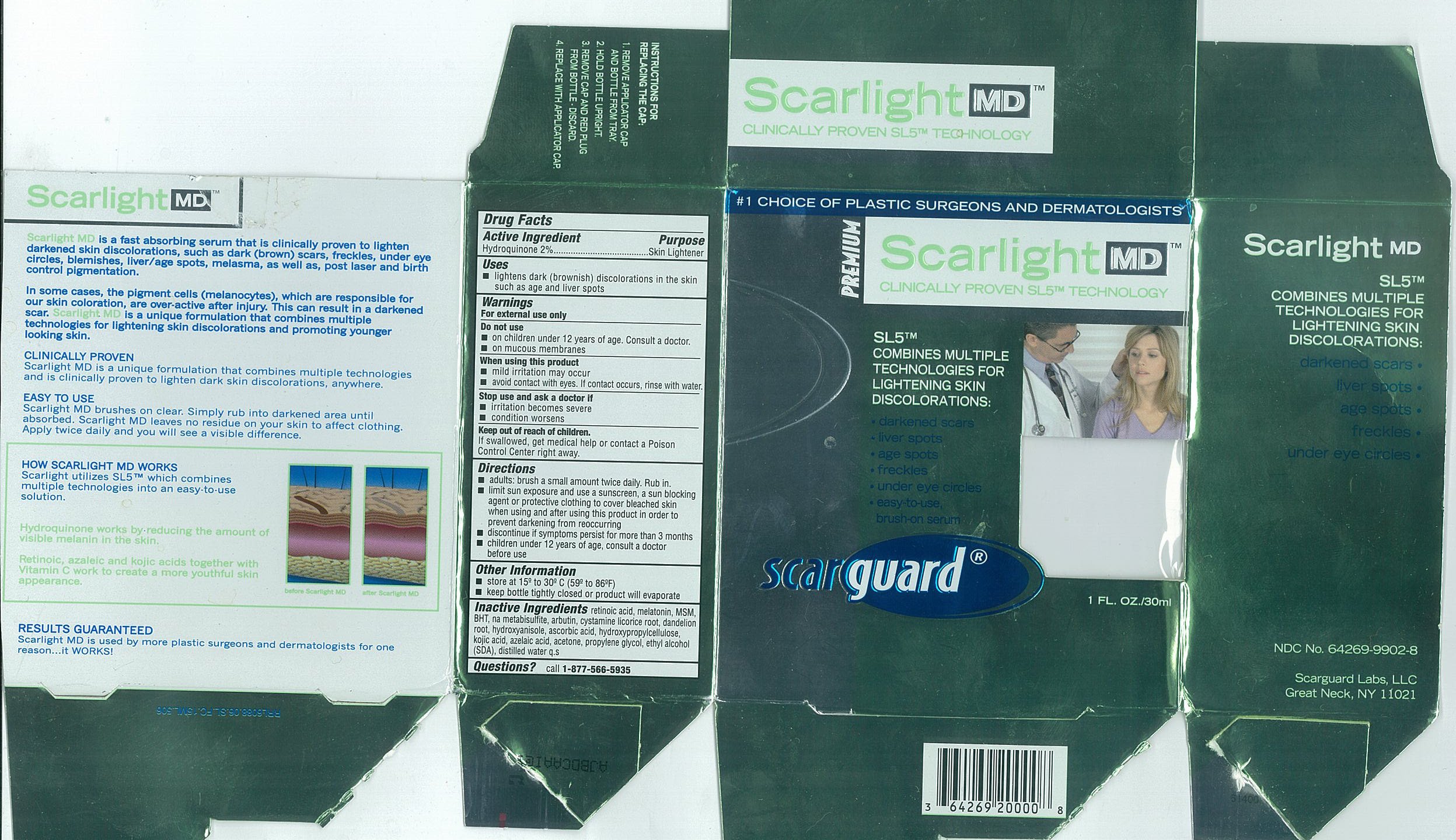
Active IngredientsHydroquinone 2%
-
PurposeSkin Lightener
-
Useslightens dark (brownish) discoloration in the skin such as age and liver spots
-
WarningsFor external use only
-
Do not useon children under 12 years of age. Consult a doctor. on mucous membranes
-
When using this productmild irritation may occur - avoid contact with eyes. If contact occurs, rinse with water.
-
Stop use and ask a doctor ifirritation becomes severe - condition worsens
-
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right away.
-
Directionsadults: brush a small amount twice daily. Rub in. limit sun exposure and use a sunscreen, a sun blocking agent or protective clothing to cover bleached skin when using and after using this ...
-
Other Informationstore at 15º to 30ºC (59 to 86ºF) keep bottle tightly closed or product will evaporate
-
Inactive Ingredientsretinoic acid, melatonin, MSM, BHT, na metabisulfite, arbutin, cystamine, licorice root, dandelion root, hydroxyanisole, ascorbic acid, hydroxypropylcellulose, kojic acid, azelaic acid, acetone ...
-
Questionscall - 1-877-566-5935
-
Carton 15mL
-
Carton 30mL
-
INGREDIENTS AND APPEARANCEProduct Information