Label: NUFLOR-S- florfenicol injection
- NDC Code(s): 0061-5581-01, 0061-5581-02
- Packager: Merck Sharp & Dohme Corp.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
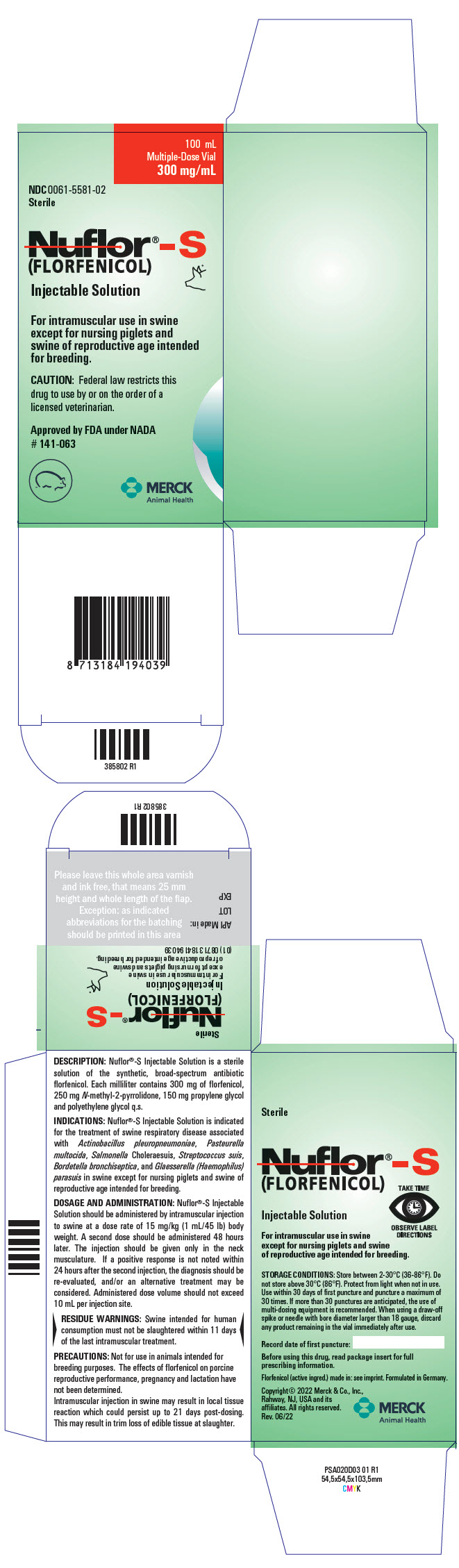
DESCRIPTION: Nuflor®-S Injectable Solution is a sterile solution of the synthetic, broad-spectrum antibiotic florfenicol. Each milliliter of sterile Nuflor®-S Injectable Solution contains 300 mg of florfenicol, 250 mg N-methyl-2-pyrrolidone (NMP), 150 mg propylene glycol and polyethylene glycol q.s.
-
VETERINARY INDICATIONS
INDICATIONS: Nuflor®-S Injectable Solution is indicated for treatment of swine respiratory disease associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Salmonella Choleraesuis, Streptococcus suis, Bordetella bronchiseptica, and Glaesserella (Haemophilus) parasuis in swine except for nursing piglets and swine of reproductive age intended for breeding.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: Nuflor®-S Injectable Solution should be administered by intramuscular injection to swine at a dose rate of 15 mg/kg (1 mL/45 lb) body weight. A second dose should be administered 48 hours later. The injection should be given only in the neck musculature. If a positive response is not noted within 24 hours after the second injection, the diagnosis should be re-evaluated, and/or an alternative treatment may be considered. Administered dose volume should not exceed 10 mL per injection site.
Nuflor®-S DOSAGE GUIDE FOR SWINE ANIMAL WEIGHT (lbs) IM Nuflor®-S DOSAGE
(1 mL/ 45 lb Body Weight)
(mL)22 0.5 45 1 90 2 135 3 180 4 225 5 270 6 -
WARNINGS
WARNINGS: NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN. This product contains materials that can be irritating to skin and eyes. Avoid direct contact with skin, eyes and clothing. In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. Consult a physician if irritation persists. Accidental injection of this product may cause local irritation. Consult a physician immediately. Reproductive and developmental toxicities have been reported in laboratory animals following high, repeated exposures to NMP. Pregnant women should wear gloves and exercise caution or avoid handling this product. The Safety Data Sheet (SDS) contains more detailed occupational safety information.
For customer service, adverse effects reporting and/or a copy of the SDS, call 1-800-211-3573. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
-
PRECAUTIONS
PRECAUTIONS: Not for use in animals intended for breeding purposes. The effects of florfenicol on porcine reproductive performance, pregnancy and lactation have not been determined.
Intramuscular injection in swine may result in local tissue reaction which could persist up to 21 days post-dosing. This may result in trim loss of edible tissue at slaughter.
- RESIDUE WARNING
- ADVERSE REACTIONS
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY: The pharmacokinetic disposition of florfenicol was evaluated in 20 pigs following a single IM injection of Nuflor®-S at the labeled dose of 15 mg/kg BW. The mean ± standard deviation maximum plasma concentration (Cmax) and the time to reach Cmax (Tmax) of florfenicol were 3.42 ± 0.82 μg/mL and 4.70 ± 2.15 hours, respectively. The mean ± standard deviation area under the drug concentration-time curve between times 0 and the last quantifiable concentration (AUC0-LOQ) and the terminal half-life (T1/2) of florfenicol were 70.34 ± 23.78 μg*hours/mL and 11.21 ± 3.73 hours, respectively.
MICROBIOLOGY: Florfenicol is a synthetic, broad-spectrum antibiotic active against many Gram-negative and Gram-positive bacteria isolated from domestic animals. It acts by binding to the 50S ribosomal subunit and inhibiting bacterial protein synthesis. Florfenicol is generally considered a bacteriostatic drug, but exhibits bactericidal activity against certain bacterial species.
In vitro activity of florfenicol has been demonstrated against commonly isolated pathogens associated with swine respiratory disease. Isolates tested were obtained from pre-treatment lung samples from representative non-enrolled pigs at each study site and post-treatment lung samples from pigs in the florfenicol-treated and saline-treated groups that died or were euthanized during the study, or were classified as treatment failures at the end of the study. The minimum inhibitory concentrations (MICs) of florfenicol for swine respiratory pathogens from clinical studies were determined using dilution methods. These susceptibility test methods were adequately controlled with the inclusion and acceptable performance of appropriate reference strains. The results are presented in Table 1.
Table 1. Florfenicol minimum inhibitory concentration (MIC) values* for indicated target pathogens isolated from a multi-site field study evaluating swine respiratory disease in the U.S. in 2001. Indicated pathogens Number of Isolates MIC50†
(μg/mL)MIC90†
(μg/mL)MIC Range
(μg/mL)Actinobacillus pleuropneumoniae 100 0.25 0.5 0.25-1 Pasteurella multocida 107 0.5 0.5 0.25-0.5 Bordetella bronchiseptica 49 2 2 0.5-4 Glaesserella parasuis 36 0.5 0.5 ≤0.12-1.0 Streptococcus suis 62 2 2 1-2 Salmonella Choleraesuis 36 4 4 2-4 EFFECTIVENESS: In a multi-site natural infection field study, a total of 620 growing pigs with clinical signs of SRD (rectal temperature of ≥ 104.5°F, and a depression score (on a scale of 0 [absent] to 3 [severe]) of ≥ 2, and a dyspnea score (on a scale of 0 [absent] to 3 [severe]) of ≥ 2) were treated with either florfenicol (15 mg/kg BW IM given on Days 0 and 2) or an equivalent volume of saline. Treatment success (rectal temperature of < 104°F, and a depression score of 0 or 1, and a dyspnea score of 0 or 1) was evaluated on Day 6. The treatment success rate was statistically significantly different (p < 0.0001) and higher in the florfenicol-treated group (72%) than in the saline-treated control group (33.1%).
-
SPL UNCLASSIFIED SECTION
ANIMAL SAFETY: A safety study was conducted in 40 healthy crossbred growing pigs. Pigs were administered florfenicol by IM injection in the neck at 1X, 3X, or 5X the labeled dose (15, 45, or 75 mg/kg BW, respectively) for 3X the labeled duration of treatment (6 injections at 48-hour intervals), or 10X the labeled dose (150 mg/kg BW) administered as two injections 48 hours apart. Test article-related diarrhea (moderate), anal swelling/erythema (mild to moderate), and injection site swelling (mild to moderate) were seen in all florfenicol-treated groups after dosing, most frequently in the 3X and 5X groups. Although these findings were considered clinically relevant, the incidence and severity in the 1X group was considered within acceptable limits. Test article-related decreases in feed and water consumption and an associated decrease in body weight were seen in the 3X and 5X groups. Test article-related changes in some serum chemistry parameters and decreased numbers of white blood cells were seen in the 3X, 5X, and/or 10X groups; the changes were generally minimal and not considered clinically significant. Most changes in drug-related, in-life parameters did not become apparent until after dosing was extended beyond the labeled duration of two injections, 48 hours apart.
Injection site irritation was evaluated in a safety study using 20 healthy crossbred growing pigs administered florfenicol at 15 mg/kg BW IM in the neck as two injections 48 hours apart. Mild injection site swelling was seen in up to approximately 32% of the pigs by 4 days post-injection and was resolved by 16 days post-injection. Gross and histopathologic evaluation showed that injection site discoloration and inflammation was present at 7 and 14 days post-injection, and absent at 21, 28, and 42 days post-injection.
-
STORAGE AND HANDLING
STORAGE CONDITIONS: Store between 2-30°C (36-86°F). Do not store above 30°C (86°F). Protect from light when not in use. Use within 30 days of first puncture and puncture a maximum of 30 times. If more than 30 punctures are anticipated, the use of multi-dosing equipment is recommended. When using a draw-off spike or needle with bore diameter larger than 18 gauge, discard any product remaining in the vial immediately after use.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 100 mL Vial Carton
100 mL
Multiple-Dose Vial
300 mg/mLNDC 0061-5581-02
SterileNuflor®-S
(FLORFENICOL)
Injectable SolutionFor intramuscular use in swine
except for nursing piglets and
swine of reproductive age intended
for breeding.CAUTION: Federal law restricts this
drug to use by or on the order of a
licensed veterinarian.Approved by FDA under NADA
# 141-063MERCK
Animal Health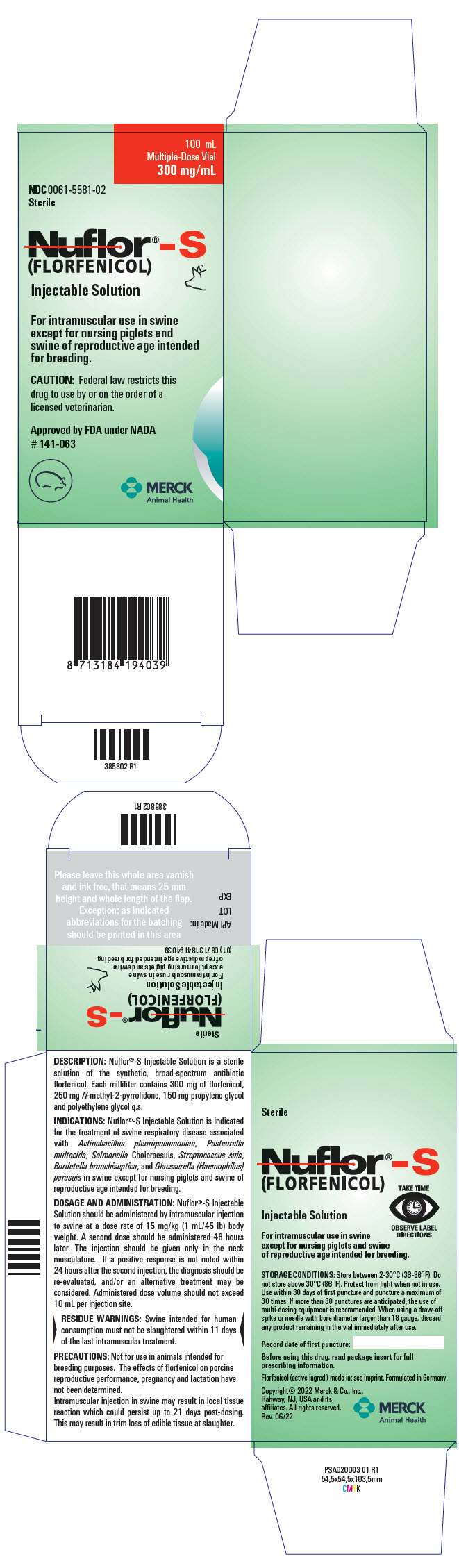
-
INGREDIENTS AND APPEARANCE
NUFLOR-S
florfenicol injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0061-5581 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLORFENICOL (UNII: 9J97307Y1H) (FLORFENICOL - UNII:9J97307Y1H) FLORFENICOL 300 mg in 1 mL Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0061-5581-02 6 in 1 CARTON 1 NDC:0061-5581-01 100 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141063 08/19/2021 Labeler - Merck Sharp & Dohme Corp. (001317601)