Label: OPTASE- white petrolatum, lanolin ointment
- NDC Code(s): 72972-003-01
- Packager: Scope Health Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Keep Out of Reach of Children
- Inactive Ingredients
- Directions
- Warnings
- Uses
-
ACTIVE INGREDIENT
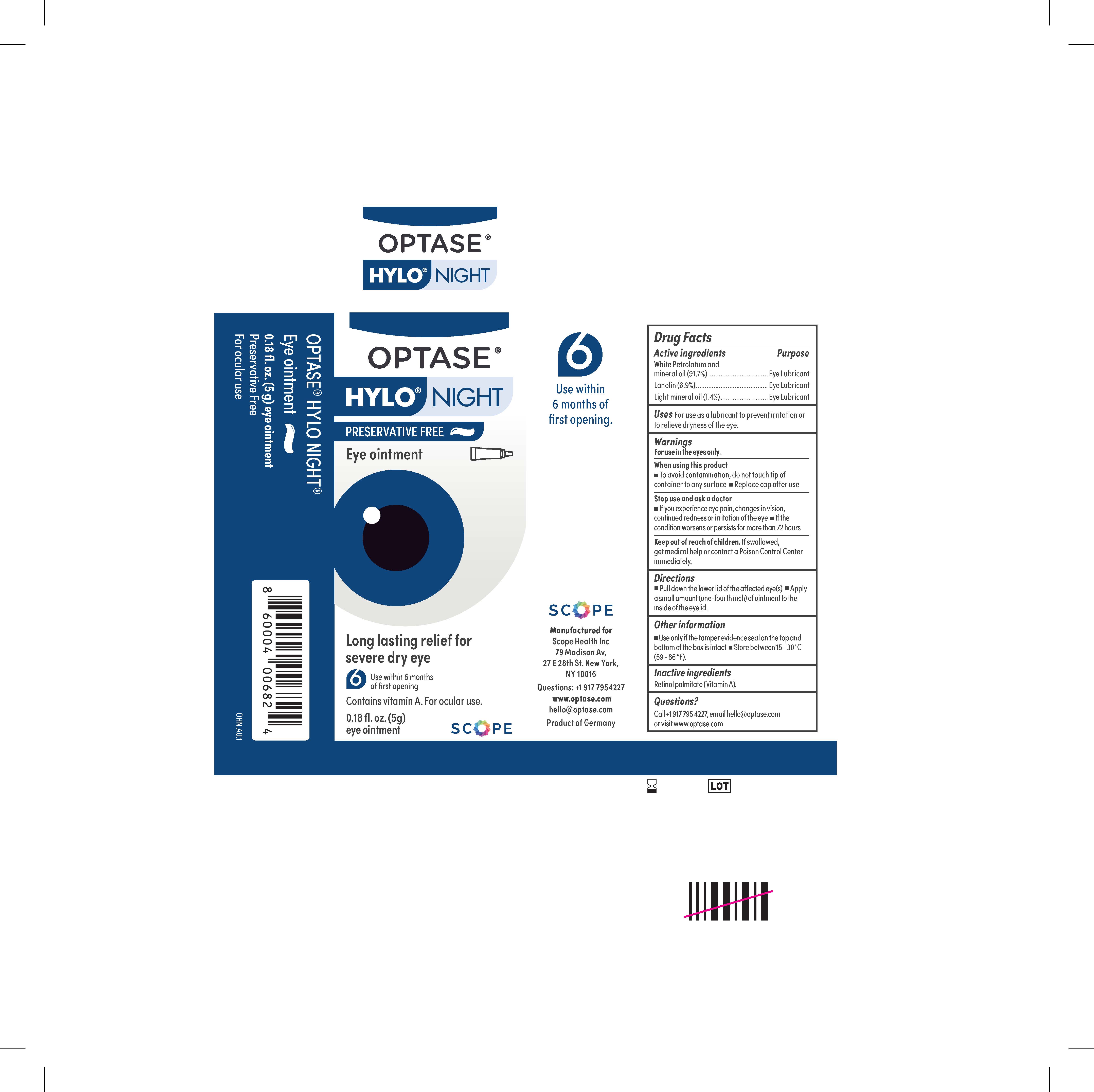
Active ingredients
White Petrolatum and
mineral oil (91.7%) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Eye Lubricant
Lanolin (6.9%) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Eye Lubricant
Light mineral oil (1.4%) . . . . . . . . . . . . . . . . . . . . . . . . . . . Eye Lubricant - PURPOSE
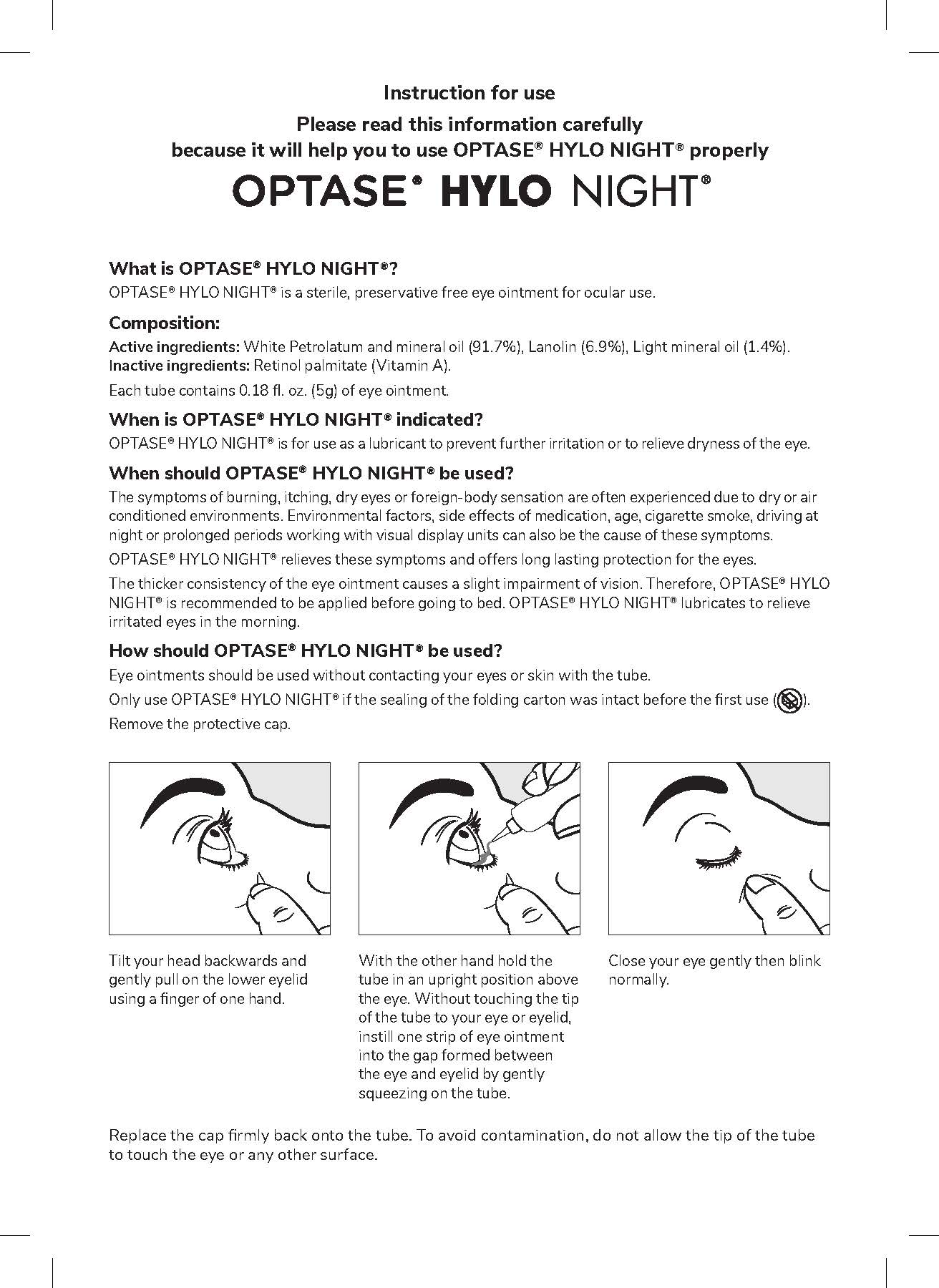
- INSTRUCTIONS FOR USE
- INSTRUCTIONS FOR USE
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OPTASE
white petrolatum, lanolin ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72972-003 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 0.9 g in 1 g LANOLIN (UNII: 7EV65EAW6H) (LANOLIN - UNII:7EV65EAW6H) LANOLIN 0.06 g in 1 g Inactive Ingredients Ingredient Name Strength LIGHT MINERAL OIL (UNII: N6K5787QVP) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72972-003-01 1 in 1 BOX 05/05/2021 1 5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 05/05/2021 Labeler - Scope Health Inc (116778693) Registrant - Regulatory Matters Consulting (080711165)