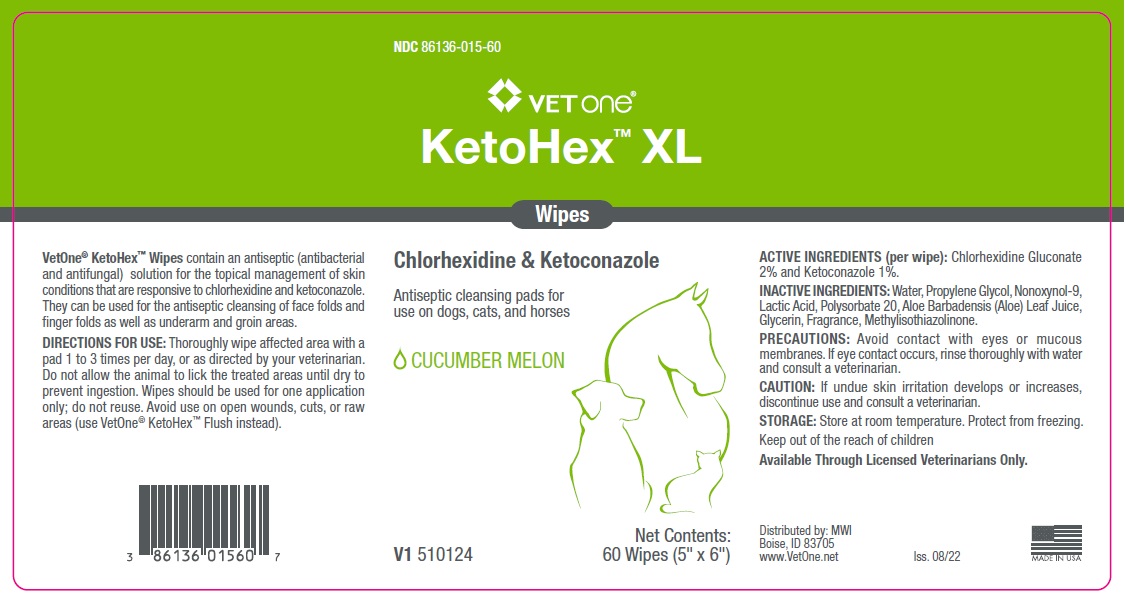
Label: VET ONE KETOHEX XL WIPES- chlorhexidine gluconate, ketoconazole cloth
- NDC Code(s): 86136-015-60
- Packager: MWI Animal Health
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 26, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS (per wipe):
- INACTIVE INGREDIENTS:
- PRECAUTIONS:
- CAUTION:
- STORAGE:
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
DIRECTIONS FOR USE:
Thoroughly wipe affected area with a pad 1 to 3 times per day, or as directed by your veterinarian. Do not allow the animal to lick the treated areas until dry to prevent ingestion. Wipes should be used for one application only; do not reuse. Avoid use on open wounds, cuts, or raw areas (use VetOne® KetoHex™ Flush instead).
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
VET ONE KETOHEX XL WIPES
chlorhexidine gluconate, ketoconazole clothProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86136-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 2 g in 100 mL KETOCONAZOLE (UNII: R9400W927I) (KETOCONAZOLE - UNII:R9400W927I) KETOCONAZOLE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) NONOXYNOL-9 (UNII: 48Q180SH9T) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) POLYSORBATE 20 (UNII: 7T1F30V5YH) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86136-015-60 60 in 1 CONTAINER 1 0.15 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/25/2022 Labeler - MWI Animal Health (019926120) Registrant - Stratford Care Usa, Inc. (036650469)