Label: LEVOLEUCOVORIN CALCIUM- levoleucovorin injection injection, solution
- NDC Code(s): 14335-340-01, 14335-341-01
- Packager: Hainan Poly Pharm. Co., Ltd.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LEVOLEUCOVORIN INJECTION safely and effectively. See full prescribing information for LEVOLEUCOVORIN INJECTION. Levoleucovorin ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Levoleucovorin injection is indicated for: rescue after high-dose methotrexate therapy in adult and pediatric patients with osteosarcoma. diminishing the toxicity associated with overdosage of ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Use Information - Levoleucovorin injection is indicated for intravenous administration only - . Do not administer intrathecally. 2.2 Co-administration ...
-
3 DOSAGE FORMS AND STRENGTHS
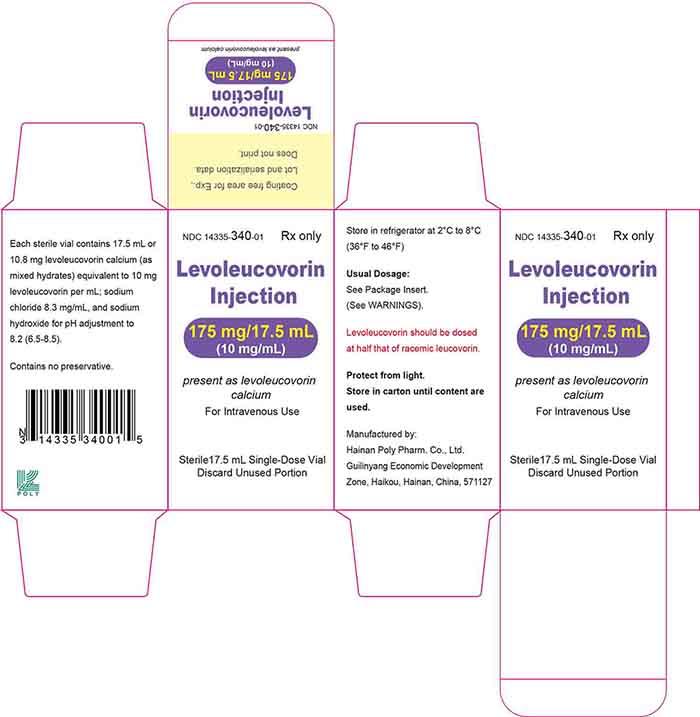
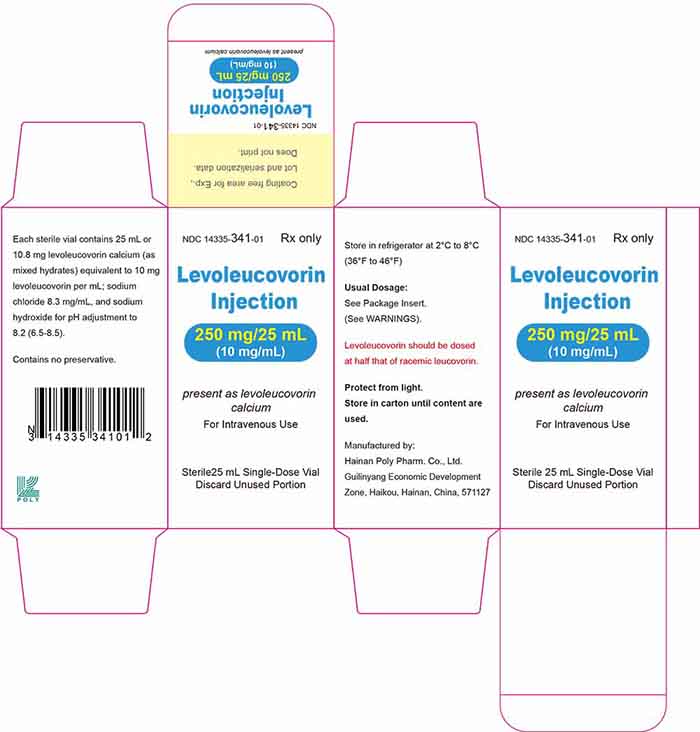
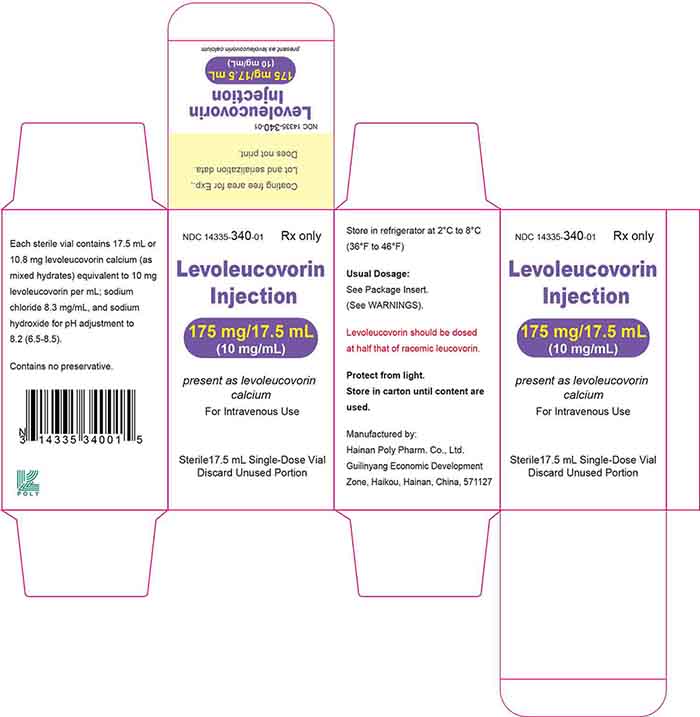
Injection: 175 mg/17.5 mL (10 mg/mL) or 250 mg/25 mL (10 mg/mL) of levoleucovorin sterile colorless to faint yellow solution in a single-dose vial.
-
4 CONTRAINDICATIONS
Levoleucovorin is contraindicated in patients who have had severe hypersensitivity to leucovorin products, folic acid or folinic acid - [see Adverse Reactions ( 6.2) ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypercalcemia - Because of the calcium content of the levoleucovorin solution, inject no more than 16 mL (160 mg of levoleucovorin) intravenously per minute. 5.2 Increased ...
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling: Hypercalcemia - [see Warnings and Precautions ( 5.1) ...
-
7 DRUG INTERACTIONS
7.1 Effects of Leucovorin Products on Other Drugs - Antiepileptic Drugs - Folic acid in large amounts may counteract the antiepileptic effect of phenobarbital, phenytoin and primidone and ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - There are limited data with levoleucovorin use in pregnant women. Animal reproduction studies have not been conducted with levoleucovorin. Levoleucovorin is ...
-
11 DESCRIPTION
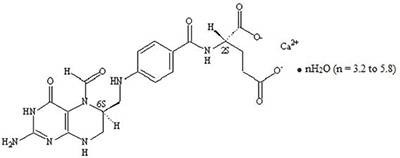
Levoleucovorin is a folate analog and the pharmacologically active levo-isomer of d,l-leucovorin. The chemical name of levoleucovorin calcium is calcium ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - High-Dose Methotrexate Therapy - Levoleucovorin is the pharmacologically active isomer of 5-formyl tetrahydrofolic acid. Levoleucovorin does not require reduction ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been conducted to evaluate the potential of levoleucovorin for carcinogenesis, mutagenesis and impairment of ...
-
14 CLINICAL STUDIES
14.1 Rescue after High-Dose Methotrexate Therapy in Patients with Osteosarcoma - The efficacy of levoleucovorin rescue following high-dose methotrexate was evaluated in 16 patients aged 6 to 21 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Levoleucovorin injection is a sterile colorless to faint yellow solution in a single-dose vial available as: 175 mg/17.5 mL (10 mg/mL) solution – NDC 14335-340-01 - 250 ...
-
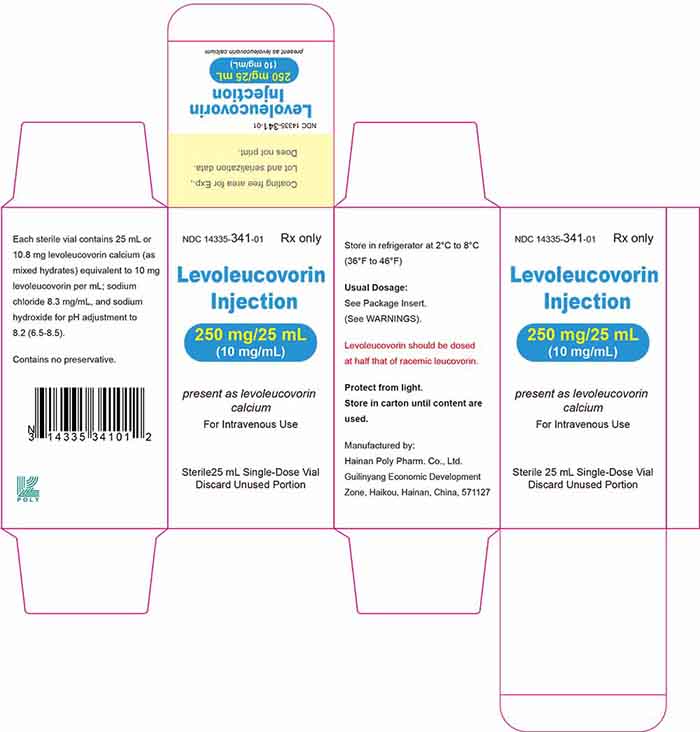
18 Principal Display Panel
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information