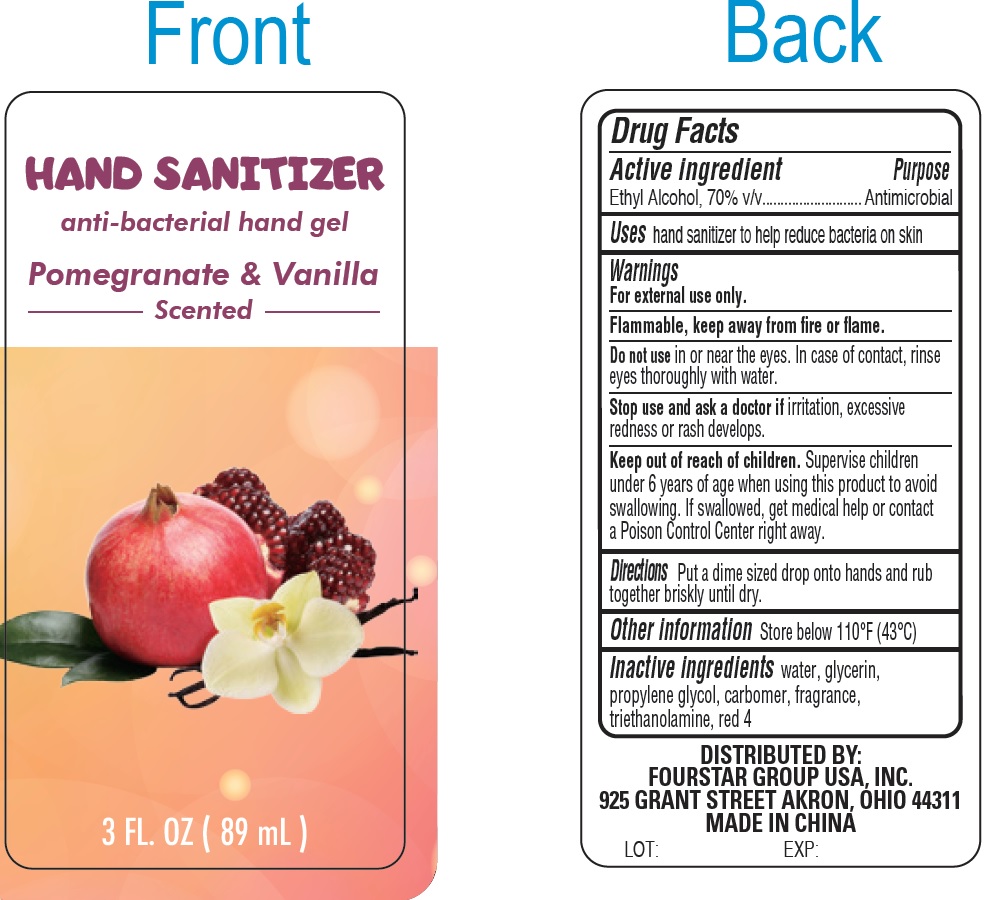
Label: POMEGRANATE AND VANILLA SCENTED HAND SANITIZER- alcohol gel
- NDC Code(s): 80684-032-01
- Packager: FOURSTAR GROUP USA, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
POMEGRANATE AND VANILLA SCENTED HAND SANITIZER
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80684-032 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) TROLAMINE (UNII: 9O3K93S3TK) FD&C RED NO. 4 (UNII: X3W0AM1JLX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80684-032-01 1 in 1 BOX 06/10/2021 1 89 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 06/10/2021 Labeler - FOURSTAR GROUP USA, INC. (140099503)