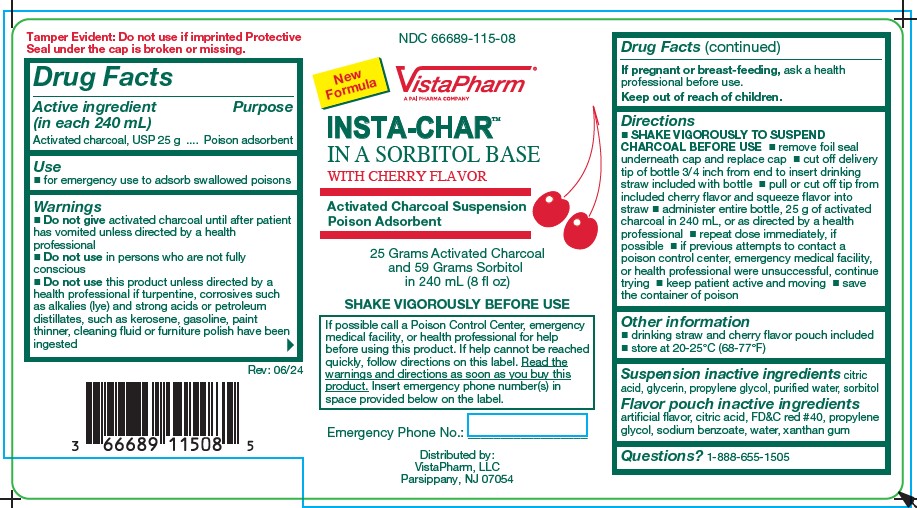
Label: INSTA-CHAR SORBITOL- poison adsorbent suspension
- NDC Code(s): 66689-115-08
- Packager: VistaPharm, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (per 240 mL)
- Purpose
- Uses
-
Warnings
Do not give
- activated charcoal until after patient has vomited unless directed by a health professional
- Do not use in persons who are not fully conscious
- Do not use this product unless directed by a health professional, if turpentine, corrosives, such as alkalies (lye) and strong acids, or petroleum distillates, such as kerosene, gasoline, paint thinner, cleaning fluid or furniture polish, have been ingested
-
Directions
- SHAKE VIGOROUSLY TO SUSPEND CHARCOAL BEFORE USE
- remove foil seal underneath cap and replace cap.
- cut off delivery tip of bottle 3/4 inch from end to insert drinking straw included with bottle.
- pull or cut off tip from included cherry flavor and squeeze flavor into straw.
- administer entire bottle, 25 g activated charcoal in 240 mL, or as directed by health professional.
- repeat dose immediately if possible.
- if previous attempts to contact a poison control center. emergency medical facility, of health professional were unsuccessful, continue trying.
- keep patient active and moving.
- save the container of poison.
- Other information
- Suspension inactive ingredients
- Questions?
-
BOXED WARNING
(What is this?)
If possible call a Poison Control Center, emergency medical facility, or health professional for help before using this product. If help cannot be reached quickly, follow directions on this label. Read the warnings and directions as soon as you buy this product. Insert emergency phone number(s) in space provided below on the label.
- Package Label
- Package Carton
-
INGREDIENTS AND APPEARANCE
INSTA-CHAR SORBITOL
poison adsorbent suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66689-115 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 50 g in 240 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66689-115-08 240 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/13/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/13/2024 Labeler - VistaPharm, LLC (048458728)